The orphan G protein-coupled receptor 161 is required for left-right patterning
- PMID: 18755178
- PMCID: PMC4639926
- DOI: 10.1016/j.ydbio.2008.08.001
The orphan G protein-coupled receptor 161 is required for left-right patterning
Abstract
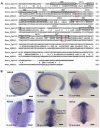
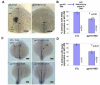
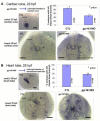
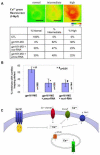
Gpr161 (also known as RE2) is an orphan G protein-coupled receptor (GPCR) that is expressed during embryonic development in zebrafish. Determining its biological function has proven difficult due to lack of knowledge regarding its natural or synthetic ligands. Here, we show that targeted knockdown of gpr161 disrupts asymmetric gene expression in the lateral plate mesoderm, resulting in aberrant looping of the heart tube. This is associated with elevated Ca(2+) levels in cells lining the Kupffer's vesicle and normalization of Ca(2+) levels, by over-expression of ncx1 or pmca-RNA, is able to partially rescue the cardiac looping defect in gpr161 knockdown embryos. Taken together, these data support a model in which gpr161 plays an essential role in left-right (L-R) patterning by modulating Ca(2+) levels in the cells surrounding the Kupffer's vesicle.
Figures


Similar articles
-
Klf8 regulates left-right asymmetric patterning through modulation of Kupffer's vesicle morphogenesis and spaw expression.J Biomed Sci. 2017 Jul 17;24(1):45. doi: 10.1186/s12929-017-0351-y. J Biomed Sci. 2017. PMID: 28716076 Free PMC article.
-
Orphan G-protein coupled receptor 22 (Gpr22) regulates cilia length and structure in the zebrafish Kupffer's vesicle.PLoS One. 2014 Oct 21;9(10):e110484. doi: 10.1371/journal.pone.0110484. eCollection 2014. PLoS One. 2014. PMID: 25335082 Free PMC article.
-
The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer's vesicle in zebrafish.Development. 2011 Jan;138(1):45-54. doi: 10.1242/dev.052985. Epub 2010 Nov 23. Development. 2011. PMID: 21098560 Free PMC article.
-
Left-right asymmetry in zebrafish.Cell Mol Life Sci. 2012 Sep;69(18):3069-77. doi: 10.1007/s00018-012-0985-6. Epub 2012 Apr 19. Cell Mol Life Sci. 2012. PMID: 22527718 Free PMC article. Review.
-
Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development.Development. 2010 Mar;137(5):681-91. doi: 10.1242/dev.038695. Development. 2010. PMID: 20147373 Free PMC article. Review.
Cited by
-
Left-right asymmetry in the light of TOR: An update on what we know so far.Biol Cell. 2015 Sep;107(9):306-18. doi: 10.1111/boc.201400094. Epub 2015 Jun 11. Biol Cell. 2015. PMID: 25943139 Free PMC article. Review.
-
Neurotransmitter map of the asymmetric dorsal habenular nuclei of zebrafish.Genesis. 2014 Jun;52(6):636-55. doi: 10.1002/dvg.22785. Epub 2014 May 8. Genesis. 2014. PMID: 24753112 Free PMC article.
-
Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics.Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):13069-13074. doi: 10.1073/pnas.1602393113. Epub 2016 Oct 31. Proc Natl Acad Sci U S A. 2016. PMID: 27799542 Free PMC article.
-
Germline GPR161 Mutations Predispose to Pediatric Medulloblastoma.J Clin Oncol. 2020 Jan 1;38(1):43-50. doi: 10.1200/JCO.19.00577. Epub 2019 Oct 14. J Clin Oncol. 2020. PMID: 31609649 Free PMC article.
-
G protein-coupled receptors in cardiac biology: old and new receptors.Biophys Rev. 2015 Mar;7(1):77-89. doi: 10.1007/s12551-014-0154-2. Epub 2015 Jan 13. Biophys Rev. 2015. PMID: 28509979 Free PMC article.
References
-
- Bergmann DC, Lee M, Robertson B, Tsou MF, Rose LS, Wood WB. Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development. 2003;130:5731–5740. - PubMed
-
- Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. - PubMed
-
- Chen JN, Fishman MC. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122:3809–3816. - PubMed
-
- Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

