Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity
- PMID: 18755202
- PMCID: PMC2590778
- DOI: 10.1016/j.neuropharm.2008.07.046
Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity
Abstract
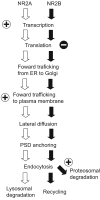
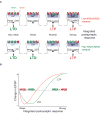
NMDA-type glutamate receptors (NMDARs) mediate many forms of synaptic plasticity. These tetrameric receptors consist of two obligatory NR1 subunits and two regulatory subunits, usually a combination of NR2A and NR2B. In the neonatal neocortex NR2B-containing NMDARs predominate, and sensory experience facilitates a developmental switch in which NR2A levels increase relative to NR2B. In this review, we clarify the roles of NR2 subunits in synaptic plasticity, and argue that a primary role of this shift is to control the threshold, rather than determining the direction, for modifying synaptic strength. We also discuss recent studies that illuminate the mechanisms regulating NR2 subunits, and suggest that the NR2A/NR2B ratio is regulated by multiple means, which may control the ratio both locally at individual synapses and globally in a cell-wide manner. Finally, we use the visual cortex as a model system to illustrate how activity-dependent modifications in the NR2A/NR2B ratio may contribute to the development of cortical functions.
Figures
References
-
- Abraham W, Bear M. Metaplasticity: The plasticity of synaptic plasticity. TINS. 1996;19:126–130. - PubMed
-
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. - PubMed
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

