Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans
- PMID: 18755348
- PMCID: PMC2748760
- DOI: 10.1016/j.jacc.2008.05.043
Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans
Abstract
Objectives: The aim of this study was to determine whether oxidative stress is increased in calcified, stenotic aortic valves and to examine mechanisms that might contribute to increased oxidative stress.
Background: Oxidative stress is increased in atherosclerotic lesions and might play an important role in plaque progression and calcification. The role of oxidative stress in valve disease is not clear.
Methods: Superoxide (dihydroethidium fluorescence and lucigenin-enhanced chemiluminescence), hydrogen peroxide H2O2 (dichlorofluorescein fluorescence), and expression and activity of pro- and anti-oxidant enzymes were measured in normal valves from hearts not suitable for transplantation and stenotic aortic valves that were removed during surgical replacement of the valve.
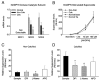
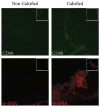
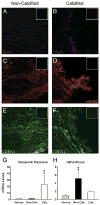
Results: In normal valves, superoxide levels were relatively low and distributed homogeneously throughout the valve. In stenotic valves, superoxide levels were increased 2-fold near the calcified regions of the valve (p < 0.05); noncalcified regions did not differ significantly from normal valves. Hydrogen peroxide levels were also markedly elevated in calcified regions of stenotic valves. Nicotinamide adenine dinucleotide phosphate oxidase activity was not increased in calcified regions of stenotic valves. Superoxide levels in stenotic valves were significantly reduced by inhibition of nitric oxide synthases (NOS), which suggests uncoupling of the enzyme. Antioxidant mechanisms were reduced in calcified regions of the aortic valve, because total superoxide dismutase (SOD) activity and expression of all 3 SOD isoforms was significantly decreased. Catalase expression also was reduced in pericalcific regions.
Conclusions: This study provides the first evidence that oxidative stress is increased in calcified regions of stenotic aortic valves from humans. Increased oxidative stress is due at least in part to reduction in expression and activity of antioxidant enzymes and perhaps to uncoupled NOS activity. Thus, mechanisms of oxidative stress differ greatly between stenotic aortic valves and atherosclerotic arteries.
Figures




Comment in
-
Oxidation, inflammation, and aortic valve calcification peroxide paves an osteogenic path.J Am Coll Cardiol. 2008 Sep 2;52(10):851-4. doi: 10.1016/j.jacc.2008.05.044. J Am Coll Cardiol. 2008. PMID: 18755349 Free PMC article. No abstract available.
References
-
- Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Am Coll Cardiol. 1998;32:1486–588. - PubMed
-
- Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–70. - PubMed
-
- Messika-Zeitoun D, Bielak LF, Peyser PA, et al. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–8. - PubMed
-
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

