The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice
- PMID: 18755353
- PMCID: PMC2633943
- DOI: 10.1016/j.jacc.2008.04.055
The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice
Abstract
Objectives: Our aim was to investigate if the peroxisome proliferator-activated receptor (PPAR)-gamma agonist pioglitazone modulates inflammation through PPARalpha mechanisms.
Background: The thiazolidinediones (TZDs) pioglitazone and rosiglitazone are insulin-sensitizing PPARgamma agonists used to treat type 2 diabetes (T2DM). Despite evidence for TZDs limiting inflammation and atherosclerosis, questions exist regarding differential responses to TZDs. In a double-blinded, placebo-controlled 16-week trial among recently diagnosed T2DM subjects (n = 34), pioglitazone-treated subjects manifested lower triglycerides and lacked the increase in soluble vascular cell adhesion molecules (sVCAM)-1 evident in the placebo group. Previously we reported PPARalpha but not PPARgamma agonists could repress VCAM-1 expression. Since both triglyceride-lowering and VCAM-1 repression characterize PPARalpha activation, we studied pioglitazone's effects via PPARalpha.
Methods: Pioglitazone effects on known PPARalpha responses--ligand binding domain activation and PPARalpha target gene expression--were tested in vitro and in vivo, including in wild-type and PPARalpha-deficient cells and mice, and compared with the effects of other PPARgamma (rosiglitazone) and PPARalpha (WY14643) agonists.
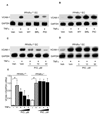
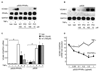
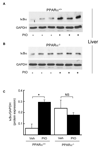
Results: Pioglitazone repressed endothelial TNFalpha-induced VCAM-1 messenger ribonucleic acid expression and promoter activity, and induced hepatic IkappaBalpha in a manner dependent on both pioglitazone exposure and PPARalpha expression. Pioglitazone also activated the PPARalpha ligand binding domain and induced PPARalpha target gene expression, with in vitro effects that were most pronounced in endothelial cells. In vivo, pioglitazone administration modulated sVCAM-1 levels and IkappaBalpha expression in wild-type but not PPARalpha-deficient mice.
Conclusions: Pioglitazone regulates inflammatory target genes in hepatic (IkappaBalpha) and endothelial (VCAM-1) settings in a PPARalpha-dependent manner. These data offer novel mechanisms that may underlie distinct TZD responses.
Figures



Comment in
-
Pharmacological differences of glitazones: does peroxisome proliferator-activated receptor-alpha activation make the difference?J Am Coll Cardiol. 2008 Sep 2;52(10):882-4. doi: 10.1016/j.jacc.2008.06.012. Epub 2008 Jul 9. J Am Coll Cardiol. 2008. PMID: 18755354 No abstract available.
References
-
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001:503–516. - PubMed
-
- Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001:365–372. - PubMed
-
- Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007:518–533. - PubMed
-
- Lindberg M, Astrup A. The role of glitazones in management of type 2 diabetes. A DREAM or a nightmare? Obes Rev. 2007:381–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

