Targeting the PIAS1 SUMO ligase pathway to control inflammation
- PMID: 18755518
- PMCID: PMC2701905
- DOI: 10.1016/j.tips.2008.07.008
Targeting the PIAS1 SUMO ligase pathway to control inflammation
Abstract
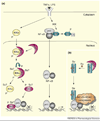
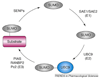
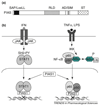
Protein sumoylation is a post-translational-modification event, in which small ubiquitin-like modifier (SUMO) is covalently attached to protein substrates by a three-step process. Sumoylation has been suggested to regulate multiple cellular processes, including inflammation. Inflammation is initiated in response to pathogenic infections, but uncontrolled inflammatory responses can lead to the development of inflammatory disorders such as rheumatoid arthritis. Recent studies indicate that proinflammatory stimuli, such as tumor necrosis factor alpha and lipopolysaccharide, can activate PIAS1 [protein inhibitor of activated STAT1 (signal transducer and activator of transcription 1)] SUMO E3 ligase through a SUMO-dependent, inhibitor of kappaB kinase alpha (IKKalpha)-mediated phosphorylation event. Activated PIAS1 is then recruited to inflammatory gene promoters to repress transcription. These findings support a hypothesis that therapies targeting the PIAS1 SUMO ligase pathway might be developed for the treatment of inflammatory disorders such as rheumatoid arthritis and atherosclerosis.
Figures

Similar articles
-
Phosphorylation of protein inhibitor of activated STAT1 (PIAS1) by MAPK-activated protein kinase-2 inhibits endothelial inflammation via increasing both PIAS1 transrepression and SUMO E3 ligase activity.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):321-9. doi: 10.1161/ATVBAHA.112.300619. Epub 2012 Nov 29. Arterioscler Thromb Vasc Biol. 2013. PMID: 23202365 Free PMC article.
-
SUMO Ligase Protein Inhibitor of Activated STAT1 (PIAS1) Is a Constituent Promyelocytic Leukemia Nuclear Body Protein That Contributes to the Intrinsic Antiviral Immune Response to Herpes Simplex Virus 1.J Virol. 2016 Jun 10;90(13):5939-5952. doi: 10.1128/JVI.00426-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27099310 Free PMC article.
-
Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity.Cell. 2007 Jun 1;129(5):903-14. doi: 10.1016/j.cell.2007.03.056. Cell. 2007. PMID: 17540171
-
Quantitative SUMO proteomics identifies PIAS1 substrates involved in cell migration and motility.Nat Commun. 2020 Feb 11;11(1):834. doi: 10.1038/s41467-020-14581-w. Nat Commun. 2020. PMID: 32047143 Free PMC article.
-
Protein inhibitor of activated STAT 1 (PIAS1) is identified as the SUMO E3 ligase of CCAAT/enhancer-binding protein β (C/EBPβ) during adipogenesis.Mol Cell Biol. 2013 Nov;33(22):4606-17. doi: 10.1128/MCB.00723-13. Epub 2013 Sep 23. Mol Cell Biol. 2013. PMID: 24061474 Free PMC article.
Cited by
-
Interaction of PIAS1 with PRRS virus nucleocapsid protein mediates NF-κB activation and triggers proinflammatory mediators during viral infection.Sci Rep. 2019 Jul 30;9(1):11042. doi: 10.1038/s41598-019-47495-9. Sci Rep. 2019. PMID: 31363150 Free PMC article.
-
SUMOylation targeting mitophagy in cardiovascular diseases.J Mol Med (Berl). 2022 Nov;100(11):1511-1538. doi: 10.1007/s00109-022-02258-4. Epub 2022 Sep 26. J Mol Med (Berl). 2022. PMID: 36163375 Review.
-
The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death.FASEB J. 2011 Oct;25(10):3416-25. doi: 10.1096/fj.11-186346. Epub 2011 Jun 15. FASEB J. 2011. PMID: 21676946 Free PMC article.
-
Multiple Functions of Protein Inhibitor of Activated STAT1 in Regulating Endothelial Cell Proliferation and Inflammation.Arterioscler Thromb Vasc Biol. 2016 Sep;36(9):1717-9. doi: 10.1161/ATVBAHA.116.308131. Arterioscler Thromb Vasc Biol. 2016. PMID: 27559144 Free PMC article. No abstract available.
-
PIAS1 Regulates Hepatitis C Virus-Induced Lipid Droplet Accumulation by Controlling Septin 9 and Microtubule Filament Assembly.Pathogens. 2021 Oct 15;10(10):1327. doi: 10.3390/pathogens10101327. Pathogens. 2021. PMID: 34684276 Free PMC article.
References
-
- Melchior F. SUMO–nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 2000;16:591–626. - PubMed
-
- Johnson ES. Protein modification by SUMO. Annu. Rev. Biochem. 2004;73:355–382. - PubMed
-
- Garaude J, et al. SUMOylation regulates the transcriptional activity of JunB in T lymphocytes. J. Immunol. 2008;180:5983–5990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

