Internalized Kv1.5 traffics via Rab-dependent pathways
- PMID: 18755741
- PMCID: PMC2614053
- DOI: 10.1113/jphysiol.2008.161570
Internalized Kv1.5 traffics via Rab-dependent pathways
Erratum in
- J Physiol. 2009 Jan 15;587(Pt 2):505
Abstract
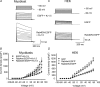
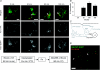
Little is known about the postinternalization trafficking of surface-expressed voltage-gated potassium channels. Here, for the first time, we investigate into which of four major trafficking pathways a voltage-gated potassium channel is targeted after internalization. In both a cardiac myoblast cell line and in HEK293 cells, channels were found to internalize and to recycle quickly. Upon internalization, Kv1.5 rapidly associated with Rab5-and Rab4-positive endosomes, suggesting that the channel is internalized via a Rab5-dependent pathway and rapidly targeted for recycling to the plasma membrane. Nevertheless, as indicated by colocalization with Rab7, a fraction of the channels are targeted for degradation. Recycling through perinuclear endosomes is limited; colocalization with Rab11 was evident only after 24 h postsurface labelling. Expression of dominant negative (DN) Rab constructs significantly increased Kv1.5 functional expression. In the myoblast line, Rab5DN increased Kv1.5 current densities to 1305 +/- 213 pA pF(-1) from control 675 +/- 81.6 pA pF(-1). Rab4DN similarly increased Kv1.5 currents to 1382 +/- 155 pA pF(-1) from the control 522 +/- 82.7 pA pF(-1) at +80 mV. Expression of the Rab7DN increased Kv1.5 currents 2.5-fold in HEK293 cells but had no significant effect in H9c2 myoblasts, and, unlike the other Rab GTPases tested, over-expression of wild-type Rab7 decreased Kv1.5 currents in the myoblast line. Densities fell to 573 +/- 96.3 pA pF(-1) from the control 869 +/- 135.5 pA pF(-1). The Rab11DN was slow to affect Kv1.5 currents but had comparable effects to other dominant negative constructs after 48 h. With the exception of Rab11DN and nocodazole, the effects of interference with microtubule-dependent trafficking by nocodazole or p50 overexpression were not additive with the Rab dominant negatives. The Rab GTPases thus constitute dynamic targets by which cells may modulate Kv1.5 functional expression.
Figures



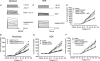











Similar articles
-
Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14681-6. doi: 10.1073/pnas.0902809106. Epub 2009 Aug 17. Proc Natl Acad Sci U S A. 2009. PMID: 19706553 Free PMC article.
-
Trafficking of an endogenous potassium channel in adult ventricular myocytes.Am J Physiol Cell Physiol. 2012 Nov 1;303(9):C963-76. doi: 10.1152/ajpcell.00217.2012. Epub 2012 Aug 22. Am J Physiol Cell Physiol. 2012. PMID: 22914645 Free PMC article.
-
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes.Circ Res. 2014 Mar 14;114(6):982-92. doi: 10.1161/CIRCRESAHA.114.302711. Epub 2014 Feb 7. Circ Res. 2014. PMID: 24508725 Free PMC article.
-
Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes.J Biol Chem. 2007 Oct 5;282(40):29612-20. doi: 10.1074/jbc.M704402200. Epub 2007 Aug 2. J Biol Chem. 2007. PMID: 17673464
-
Remodeling of Ion Channel Trafficking and Cardiac Arrhythmias.Cells. 2021 Sep 14;10(9):2417. doi: 10.3390/cells10092417. Cells. 2021. PMID: 34572065 Free PMC article. Review.
Cited by
-
Kif5b is an essential forward trafficking motor for the Kv1.5 cardiac potassium channel.J Physiol. 2009 Oct 1;587(Pt 19):4565-74. doi: 10.1113/jphysiol.2009.178442. Epub 2009 Aug 12. J Physiol. 2009. PMID: 19675065 Free PMC article.
-
Electrophysiology of reactive oxygen production in signaling endosomes.Antioxid Redox Signal. 2009 Jun;11(6):1335-47. doi: 10.1089/ars.2008.2448. Antioxid Redox Signal. 2009. PMID: 19207039 Free PMC article. Review.
-
Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14681-6. doi: 10.1073/pnas.0902809106. Epub 2009 Aug 17. Proc Natl Acad Sci U S A. 2009. PMID: 19706553 Free PMC article.
-
Antidepressant-induced ubiquitination and degradation of the cardiac potassium channel hERG.J Biol Chem. 2011 Sep 30;286(39):34413-25. doi: 10.1074/jbc.M111.254367. Epub 2011 Aug 9. J Biol Chem. 2011. PMID: 21832094 Free PMC article.
-
Intravascular pressure enhances the abundance of functional Kv1.5 channels at the surface of arterial smooth muscle cells.Sci Signal. 2015 Aug 18;8(390):ra83. doi: 10.1126/scisignal.aac5128. Sci Signal. 2015. PMID: 26286025 Free PMC article.
References
-
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. - PubMed
-
- Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. - PubMed
-
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

