Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus
- PMID: 18755745
- PMCID: PMC2652150
- DOI: 10.1113/jphysiol.2008.159665
Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus
Abstract
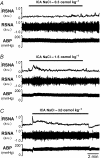
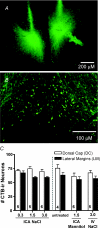
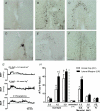
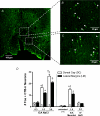
Body fluid hyperosmolality has long been known to elicit homeostatic responses that range from drinking to inhibition of salt appetite to release of neurohypohyseal hormones (i.e. vasopressin and oxytocin). More recently, it has been recognized that hyperosmolality is capable of also provoking a significant increase of sympathetic nerve activity (SNA). It has been reported that neurones in the forebrain organum vasculosum laminae terminalis (OVLT) and hypothalamic paraventricular nucleus (PVN) each contribute significantly to this response. Here we sought to determine if sympathoexcitatory levels of hyperosmolality activate specifically those OVLT neurones that form a monosynaptic pathway to the PVN. First, we established in anaesthetized rats that graded concentrations of hypertonic NaCl (1.5 and 3.0 osmol kg(-1)) elicit graded increases of renal SNA (RSNA) when infused at a rate of 0.1 ml min(-1) through an internal carotid artery (ICA) - the major vascular supply of the forebrain. Next, infusions were performed in conscious rats in which OVLT neurones projecting to the PVN (OVLT-PVN) were retrogradely labelled with cholera toxin subunit B (CTB). Immunostaining of the immediate early gene product Fos and CTB was performed to quantify osmotic activation of OVLT-PVN neurones. ICA infusions of hypertonic NaCl and mannitol each significantly (P < 0.01-0.001) increased the number of Fos immunoreactive (Fos-ir) neuronal nuclei in the dorsal cap (DC) and lateral margins (LM) of OVLT. In the LM, infusions of 1.5 and 3.0 osmol kg(-1) NaCl produced similar increases in the number of Fos-ir neurones. In the DC, these infusions produced graded increases in Fos expression. Among OVLT neurones with axons projecting directly to the PVN (i.e. CTB-ir), graded hypertonic NaCl infusions again produced graded increases in Fos expression and this was observed in both the DC and LM. Although the DC and LM contained a similar number of OVLT-PVN neurones, the proportion of such neurones that expressed Fos-ir in responses to ICA hypertonic NaCl infusions was greater in the DC (P < 0.001). These findings support the conclusion that PVN-projecting neurones in the DC and LM of OVLT could participate in behavioural, neuroendocrine, and sympathetic nervous system responses to body fluid hyperosmolality.
Figures

Comment in
-
Pathways to hypertension.J Physiol. 2008 Nov 1;586(21):5033. doi: 10.1113/jphysiol.2008.163014. J Physiol. 2008. PMID: 18978156 Free PMC article. No abstract available.
References
-
- Allen AM, Oldfield BJ, Giles ME, Paxinos G, McKinley MJ, Mendelsohn FAO. Localization of angiotensin receptors in the nervous system. In: Quirion R, Björklund A, Hökfelt T, editors. Peptide Receptors. Vol. 16. Elsevier Science; 2000. pp. 79–124. part 1.
-
- Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience. 2000;100:539–547. - PubMed
-
- Bealer SL. Increased dietary sodium inhibits baroreflex-induced bradycardia during acute sodium loading. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1211–R1219. - PubMed
-
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

