Molecular mechanisms supporting a paracrine role of GABA in rat adrenal medullary cells
- PMID: 18755746
- PMCID: PMC2614061
- DOI: 10.1113/jphysiol.2008.158709
Molecular mechanisms supporting a paracrine role of GABA in rat adrenal medullary cells
Abstract
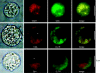
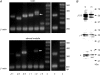
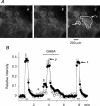
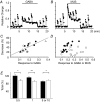
GABA is known to produce membrane depolarization and secretion in adrenal medullary (AM) cells in various species. However, whether the GABAergic system is intrinsic or extrinsic or both in the adrenal medulla and the role that GABA plays are controversial. Therefore, these issues were addressed by combining a biochemical and functional analysis. Glutamic acid decarboxylase (GAD), a GABA synthesizing enzyme, and vesicular GABA transporter (VGAT) were expressed in rat AM cells at the mRNA and protein levels, and the adrenal medulla had no nerve fibre-like structures immunoreactive to an anti-GAD Ab. The double staining for VGAT and chromogranin A indicates that GABA was stored in chromaffin granules. The alpha1, alpha3, beta2/3, gamma2 and delta subunits of GABA(A) receptors were identified in AM cells at the mRNA and protein levels. Pharmacological properties of GABA-induced Cl(-) currents, immunoprecipitation experiments and immunocytochemistry indicated the expression of not only gamma2-, but also delta-containing GABA(A) receptors, which have higher affinities for GABA and neurosteroids. Expression of GATs, which are involved in the clearance of GABA at GABAergic synapses, were conspicuously suppressed in the adrenal medulla, compared with expression levels of GABA(A) receptors. Increases in Ca(2+) signal in AM cells evoked trans-synaptically by nerve stimulation were suppressed during the response to GABA, and this suppression was attributed to the shunt effect of the GABA-induced increase in conductance. Overall Ca(2+) responses to electrical stimulation and GABA in AM cells were larger or smaller than those to electrical stimulation alone, depending on the frequency of stimulation. The results indicate that GABA functions as a paracrine in rat AM cells and this function may be supported by the suppression of GAT expression and the expression of not only gamma2-, but also delta-GABA(A) receptors.
Figures






Similar articles
-
Storage of GABA in chromaffin granules and not in synaptic-like microvesicles in rat adrenal medullary cells.J Neurochem. 2010 Jul;114(2):617-26. doi: 10.1111/j.1471-4159.2010.06792.x. Epub 2010 Apr 30. J Neurochem. 2010. PMID: 20477909
-
Regulation of α3-containing GABAA receptors in guinea-pig adrenal medullary cells by adrenal steroids.Neuroscience. 2013 Dec 3;253:245-55. doi: 10.1016/j.neuroscience.2013.08.046. Epub 2013 Sep 3. Neuroscience. 2013. PMID: 24012744
-
Mechanisms for establishment of GABA signaling in adrenal medullary chromaffin cells.J Neurochem. 2021 Jul;158(2):153-168. doi: 10.1111/jnc.15345. Epub 2021 Mar 24. J Neurochem. 2021. PMID: 33704788
-
GABA Signaling and Neuroactive Steroids in Adrenal Medullary Chromaffin Cells.Front Cell Neurosci. 2016 Apr 18;10:100. doi: 10.3389/fncel.2016.00100. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27147972 Free PMC article. Review.
-
GABAA receptor: a unique modulator of excitability, Ca2+ signaling, and catecholamine release of rat chromaffin cells.Pflugers Arch. 2018 Jan;470(1):67-77. doi: 10.1007/s00424-017-2080-1. Epub 2017 Nov 3. Pflugers Arch. 2018. PMID: 29101464 Review.
Cited by
-
Post-translational modification of glutamic acid decarboxylase 67 by intermittent hypoxia: evidence for the involvement of dopamine D1 receptor signaling.J Neurochem. 2010 Dec;115(6):1568-78. doi: 10.1111/j.1471-4159.2010.07063.x. Epub 2010 Nov 19. J Neurochem. 2010. PMID: 20969567 Free PMC article.
-
Developmental Changes in Gap Junction Expression in Rat Adrenal Medullary Chromaffin Cells.Acta Histochem Cytochem. 2024 Dec 20;57(6):189-197. doi: 10.1267/ahc.24-00033. Epub 2024 Dec 11. Acta Histochem Cytochem. 2024. PMID: 39776934 Free PMC article.
-
Muscarinic Receptor Stimulation Does Not Inhibit Voltage-dependent Ca2+ Channels in Rat Adrenal Medullary Chromaffin Cells.Acta Histochem Cytochem. 2023 Aug 30;56(4):67-75. doi: 10.1267/ahc.23-00042. Epub 2023 Aug 23. Acta Histochem Cytochem. 2023. PMID: 37680574 Free PMC article.
-
Modulation of spontaneous intracellular Ca²⁺ fluctuations and spontaneous cholinergic transmission in rat chromaffin cells in situ by endogenous GABA acting on GABAA receptors.Pflugers Arch. 2016 Feb;468(2):351-65. doi: 10.1007/s00424-015-1744-y. Epub 2015 Oct 21. Pflugers Arch. 2016. PMID: 26490458
-
Nerve growth factor-induced endocytosis of TWIK-related acid-sensitive K⁺ 1 channels in adrenal medullary cells and PC12 cells.Pflugers Arch. 2013 Jul;465(7):1051-64. doi: 10.1007/s00424-013-1222-3. Epub 2013 Feb 2. Pflugers Arch. 2013. PMID: 23377568
References
-
- Adachi J, Mizoi Y. Acetaldehyde-mediated alcohol sensitivity and elevation of plasma catecholamine in man. Jpn J Pharmacol. 1983;33:531–539. - PubMed
-
- Akinci MK, Schofield PR. Widespread expression of GABAA receptor subunits in peripheral tissues. Neurosci Res. 1999;35:145–153. - PubMed
-
- Annaert WG, Llona I, Backer AC, Jacob WA, De Potter WP. Catecholamines are present in a synaptic-like microvesicle-enriched fraction from bovine adrenal medulla. J Neurochem. 1993;60:1746–1754. - PubMed
-
- Araujo F, Ruano D, Vitorica J. Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol. 1998;347:347–353. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

