Regulation of smooth muscle contractility by the epithelium in rat vas deferens: role of ATP-induced release of PGE2
- PMID: 18755753
- PMCID: PMC2614070
- DOI: 10.1113/jphysiol.2008.154096
Regulation of smooth muscle contractility by the epithelium in rat vas deferens: role of ATP-induced release of PGE2
Abstract
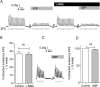
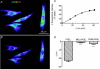
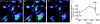
Recent studies suggest that the epithelium might modulate the contractility of smooth muscle. However, the mechanisms underlying this regulation are unknown. The present study investigated the regulation of smooth muscle contraction by the epithelium in rat vas deferens and the possible factor(s) involved. Exogenously applied ATP inhibited electrical field stimulation (EFS)-evoked smooth muscle contraction in an epithelium-dependent manner. As the effects of ATP on smooth muscle contractility were abrogated by inhibitors of prostaglandin synthesis, but not by those of nitric oxide synthesis, prostaglandins might mediate the effects of ATP. Consistent with this idea, PGE(2) inhibited EFS-evoked smooth muscle contraction independent of the epithelium, while ATP and UTP induced the release of PGE(2) from cultured rat vas deferens epithelial cells, but not smooth muscle cells. The ATP-induced PGE(2) release from vas deferens epithelial cells was abolished by U73122, an inhibitor of phospholipase C (PLC) and BAPTA AM, a Ca(2+) chelator. ATP also transiently increased [Ca(2+)](i) in vas deferens epithelial cells. This effect of ATP on [Ca(2+)](i) was independent of extracellular Ca(2+), but abolished by the P2 receptor antagonist RB2 and U73122. In membrane potential measurements using a voltage-sensitive dye, PGE(2), but not ATP, hyperpolarized vas deferens smooth muscle cells and this effect of PGE(2) was blocked by MDL12330A, an adenylate cyclase inhibitor, and the chromanol 293B, a blocker of cAMP-dependent K(+) channels. Taken together, our results suggest that ATP inhibition of vas deferens smooth muscle contraction is epithelium dependent. The data also suggest that ATP activates P2Y receptor-coupled Ca(2+) mobilization leading to the release of PGE(2) from epithelial cells, which in turn activates cAMP-dependent K(+) channels in smooth muscle cells leading to the hyperpolarization of membrane voltage and the inhibition of vas deferens contraction. Thus, the present findings suggest a novel regulatory mechanism by which the epithelium regulates the contractility of smooth muscle.
Figures





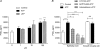

Comment in
-
Purinergic agonists flex vas deferens muscle.J Physiol. 2008 Nov 15;586(22):5287. doi: 10.1113/jphysiol.2008.164350. J Physiol. 2008. PMID: 19011131 Free PMC article. No abstract available.
References
-
- Aitken RJ, Kelly RW. Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil. 1985;73:139–146. - PubMed
-
- Balzary RW, Cocks TM. Lipopolysaccharide induces epithelium- and prostaglandin E2-dependent relaxation of mouse isolated trachea through activation of cyclooxygenase (COX)-1 and COX-2. J Pharmacol Exp Ther. 2006;317:806–812. - PubMed
-
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. - PubMed
-
- Brauner T, Hulser DF, Strasser RJ. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim Biophys Acta. 1984;771:208–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

