Genes associated with membrane-initiated signaling of estrogen and energy homeostasis
- PMID: 18755790
- PMCID: PMC2613047
- DOI: 10.1210/en.2008-0769
Genes associated with membrane-initiated signaling of estrogen and energy homeostasis
Abstract
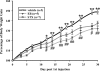
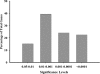
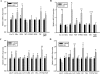
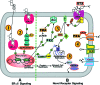
During the reproductive cycle, fluctuations in circulating estrogens affect multiple homeostatic systems controlled by hypothalamic neurons. Two of these neuronal populations are arcuate proopiomelanocortin and neuropeptide Y neurons, which control energy homeostasis and feeding. Estradiol modulates these neurons either through the classical estrogen receptors (ERs) to control gene transcription or through a G protein-coupled receptor (mER) activating multiple signaling pathways. To differentiate between these two divergent ER-mediated mechanisms and their effects on homeostasis, female guinea pigs were ovariectomized and treated systemically with vehicle, estradiol benzoate (EB) or STX, a selective mER agonist, for 4 wk, starting 7 d after ovariectomy. Individual body weights were measured after each injection day for 28 d, at which time the animals were euthanized, and the arcuate nucleus was microdissected. As predicted, the body weight gain was significantly lower for EB-treated females after d 5 and for STX-treated females after d 12 compared with vehicle-treated females. Total arcuate RNA was extracted from all groups, but only the vehicle and STX-treated samples were prepared for gene microarray analysis using a custom guinea pig gene microarray. In the arcuate nucleus, 241 identified genes were significantly regulated by STX, several of which were confirmed by quantitative real-time PCR and compared with EB-treated groups. The lower weight gain of EB-treated and STX-treated females suggests that estradiol controls energy homeostasis through both ERalpha and mER-mediated mechanisms. Genes regulated by STX indicate that not only does it control neuronal excitability but also alters gene transcription via signal transduction cascades initiated from mER activation.
Figures
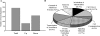
Similar articles
-
Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms.J Neuroendocrinol. 2009 Feb;21(2):141-50. doi: 10.1111/j.1365-2826.2008.01814.x. Epub 2008 Dec 6. J Neuroendocrinol. 2009. PMID: 19076267 Free PMC article. Review.
-
Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus.Endocrinology. 2007 Oct;148(10):4937-51. doi: 10.1210/en.2007-0605. Epub 2007 Jun 26. Endocrinology. 2007. PMID: 17595223
-
Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis.Endocrinology. 2010 Oct;151(10):4926-37. doi: 10.1210/en.2010-0573. Epub 2010 Aug 4. Endocrinology. 2010. PMID: 20685867 Free PMC article.
-
Adaptation of arcuate insulin receptor, estrogen receptor-alpha, estrogen receptor-beta, and type-II glucocorticoid receptor gene profiles to chronic intermediate insulin-induced hypoglycemia in estrogen-treated ovariectomized female rats.J Mol Neurosci. 2010 Jun;41(2):304-9. doi: 10.1007/s12031-009-9314-4. Epub 2009 Dec 2. J Mol Neurosci. 2010. PMID: 19953342
-
Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus.J Neuroendocrinol. 2009 Mar;21(4):263-70. doi: 10.1111/j.1365-2826.2009.01846.x. J Neuroendocrinol. 2009. PMID: 19187465 Free PMC article. Review.
Cited by
-
Estrogenic Action in Stress-Induced Neuroendocrine Regulation of Energy Homeostasis.Cells. 2022 Mar 3;11(5):879. doi: 10.3390/cells11050879. Cells. 2022. PMID: 35269500 Free PMC article. Review.
-
Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms.J Neuroendocrinol. 2009 Feb;21(2):141-50. doi: 10.1111/j.1365-2826.2008.01814.x. Epub 2008 Dec 6. J Neuroendocrinol. 2009. PMID: 19076267 Free PMC article. Review.
-
17Beta-estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor alpha and estrogen receptor beta.J Comp Neurol. 2009 Jan 20;512(3):347-58. doi: 10.1002/cne.21901. J Comp Neurol. 2009. PMID: 19003958 Free PMC article.
-
Identification of a novel AIFM1 variant from a Chinese family with auditory neuropathy.Front Genet. 2022 Nov 21;13:1064823. doi: 10.3389/fgene.2022.1064823. eCollection 2022. Front Genet. 2022. PMID: 36479253 Free PMC article.
-
RNA-sequencing of AVPV and ARH reveals vastly different temporal and transcriptomic responses to estradiol in the female rat hypothalamus.PLoS One. 2021 Aug 18;16(8):e0256148. doi: 10.1371/journal.pone.0256148. eCollection 2021. PLoS One. 2021. PMID: 34407144 Free PMC article.
References
-
- Priest CA, Roberts JL 2000 Estrogen and tamoxifen differentially regulate β-endorphin and cFos expression and neuronal colocalization in the arcuate nucleus of the rat. Neuroendocrinology 72:293–305 - PubMed
-
- Pelletier G, Li S, Luu-The V, Labrie F 2007 Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol 19:426–431 - PubMed
-
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S 2001 Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142:4751–4757 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DA007262/DA/NIDA NIH HHS/United States
- NS38809/NS/NINDS NIH HHS/United States
- 1F32 DK079508/DK/NIDDK NIH HHS/United States
- R01 NS043330/NS/NINDS NIH HHS/United States
- DK 68098/DK/NIDDK NIH HHS/United States
- 5T32 DA07262/DA/NIDA NIH HHS/United States
- R56 NS038809/NS/NINDS NIH HHS/United States
- R01 DK057574/DK/NIDDK NIH HHS/United States
- R01 DK068098/DK/NIDDK NIH HHS/United States
- DK 57574/DK/NIDDK NIH HHS/United States
- F32 DK079508/DK/NIDDK NIH HHS/United States
- NS 43330/NS/NINDS NIH HHS/United States
- R01 NS038809/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

