Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism
- PMID: 18755894
- PMCID: PMC2533243
- DOI: 10.1073/pnas.0803429105
Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism
Abstract
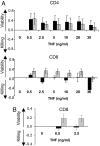
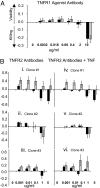
Human autoimmune (AI) diseases are difficult to treat, because immunosuppressive drugs are nonspecific, produce high levels of adverse effects, and are not based on mechanistic understanding of disease. Destroying the rare autoreactive T lymphocytes causing AI diseases would improve treatment. In animal models, TNF selectively kills autoreactive T cells, thereby hampering disease onset or progression. Here, we seek to determine, in fresh human blood, whether TNF or agonists of TNF selectively kill autoreactive T cells, while sparing normal T cells. We isolated highly pure CD4 or CD8 T cells from patients with type 1 diabetes (n = 675), other AI diseases, and healthy controls (n = 512). Using two cell death assays, we found that a subpopulation of CD8, but not CD4, T cells in patients' blood was vulnerable to TNF or TNF agonist-induced death. One agonist for the TNFR2 receptor exhibited a dose-response pattern of killing. In type 1 diabetes, the subpopulation of T cells susceptible to TNF or TNFR2 agonist-induced death was traced specifically to autoreactive T cells to insulin, a known autoantigen. Other activated and memory T cell populations were resistant to TNF-triggered death. This study shows that autoreactive T cells, although rare, can be selectively destroyed in isolated human blood. TNF and a TNFR2 agonist may offer highly targeted therapies, with the latter likely to be less systemically toxic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

