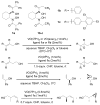
Vanadium-catalyzed enantioselective desymmetrization of meso secondary allylic alcohols and homoallylic alcohols
- PMID: 18756576
- PMCID: PMC3443975
- DOI: 10.1002/anie.200802523
Vanadium-catalyzed enantioselective desymmetrization of meso secondary allylic alcohols and homoallylic alcohols
Abstract
Vanadium-catalyzed epoxidation has extended substrate scope. In addition to various bis-allylic alcohols, bis-homoallylic alcohols can also be desymmetrized using our Vanadium-Bis-hydroxamic acid complexes.
Figures
References
-
-
For recent reviews, see: Katsuki T. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Springer; Berlin: 1999. p. 621.Katsuki T, Martin VS. Org React. 1996;48:1.Keith JM, Larrow JF, Jacobsen EN. Adv Synth Catal. 2001;343:5.Adam W, Malisch W, Roschmann KJ, Saha-Möller CR, Schenk WA. J Organomet Chem. 2002;661:3.Shi Y. Acc Chem Res. 2004;37:488.Shi Y, Frohn M. Synthesis. 2000;14:1979.
-
-
- Zhang W, Basak A, Kosugi Y, Hoshino Y, Yamamoto H. Angew Chem Int Ed. 2005;44:4389. - PubMed
-
-
For studies toward desymmetrization via olefin epoxidation, see: Hatakeyama S, Sakurai K, Takano S. J Chem Soc, Chem Commun. 1985:1759.Schreiber SL, Schreiber TS, Smith DB. J Am Chem Soc. 1987;109:1525.Schreiber SL, Goulet MT, Schulte G. J Am Chem Soc. 1987;109:4718.Kramer R, Berkenbusch T, Brückner R. Adv Synth Catal. 2008;350:1131.
-
-
-
For synthetic applications of desymmetrization via Sharpless Epoxidation, see: Kobayashi Y, Kato N, Shimazaki T, Sato F. Tetrahedron Letters. 1988;29:6297.Hatakeyama S, Sakurai K, Numata H, Ochi N, Takano S. J Am Chem Soc. 1988;110:5201.Smith DB, Zhaoyin W, Schreiber SL. Tetrahedron. 1990;46:4793.Berkenbusch T, Brückner R. Synlett. 2003:1813.Kramer R, Brückner R. Synlett. 2006:0033.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous