Permeabilities of rebamipide via rat intestinal membranes and its colon specific delivery using chitosan capsule as a carrier
- PMID: 18756602
- PMCID: PMC2739947
- DOI: 10.3748/wjg.14.4928
Permeabilities of rebamipide via rat intestinal membranes and its colon specific delivery using chitosan capsule as a carrier
Abstract
Aim: To investigate the permeability characteristics of rebamipide across intestinal mucosa, and examine the effects of some absorption enhancers on the permeability across the colonic tissue. Another purpose is to demonstrate the colon-specific delivery of rebamipide with or without absorption enhancers using chitosan capsule as a carrier.
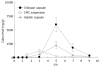
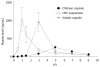
Methods: The permeability of rebamipide was evaluated using an in vitro diffusion chamber system, and the effects of some absorption enhancers on the permeability via colon were further investigated. The release of rebamipide from chitosan or gelatin capsule was studied by Japan Pharmacopoeia rotating basket method. The colonic and plasma concentrations were analyzed by high performance liquid chromatography (HPLC) to evaluate colon-targeting action after oral administration of various dosage forms, and rebamipide with absorption enhancers in chitosan dosage forms.
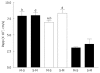
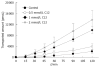
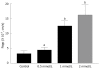
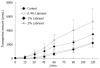
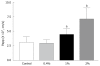
Results: The permeability of rebamipide across the jejunal or ileal membranes was higher than the colonic membranes. Both sodium laurate (C12) and labrasol significantly increased permeability across the colon membranes. On the other hand, the release of rebamipide from chitosan capsule was less than 10% totally within 6 h. The area under concentration-time profile of drug in the colon mucosa using chitosan capsules (AUCLI, 16011.2 ng x h/g) was 2.5 times and 4.4 times greater than using gelatin capsules and CMC suspension, respectively. Meanwhile, the area under concentration-time profile of drug in the plasma (AUCPL) was 1016.0 ng x h/mL for chitosan capsule, 1887.9 ng x h/mL for CMC suspension p and 2163.5 ng x h/mL for gelatin capsule. Overall, both AUCLI and AUCPL were increased when C12 was co-administrated, but the increase of AUCLI was much greater; the drug delivery index (DDI) was more than 1 compared with simple chitosan capsule group.
Conclusion: There was a regional difference in the permeability of Rabamipide across the jejunum, ileum and the colon, and passive diffusion seems to be one of the major transport mechanisms of rebamipide. Absorption enhancers can increase the permeability of rebamipide across the colon tissue significantly. In addition, chitosan capsule may be a useful carrier to deliver rebamipide to the colon specifically and the co-administration of C12 with rebamipide may also be very useful in local treatment.
Figures




References
-
- Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H, Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur J Pharmacol. 1987;142:23–29. - PubMed
-
- Liu YJ, Mei ZC. Progress of Rebamipide in Pharmacological Mechanism and Clinical Applications. Yaoxue Zhuanlun. 2005;14:24–26.
-
- Niwa Y, Nakamura M, Ohmiya N, Maeda O, Ando T, Itoh A, Hirooka Y, Goto H. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study. J Gastroenterol. 2008;43:270–276. - PubMed
-
- Kishimoto S, Haruma K, Tari A, Sakurai K, Nakano M, Nakagawa Y. Rebamipide, an antiulcer drug, prevents DSS-induced colitis formation in rats. Dig Dis Sci. 2000;45:1608–1616. - PubMed
-
- Makiyama K, Takeshima F, Kawasaki H, Zea-Iriarte WL. Anti-inflammatory effect of rebamipide enema on proctitis type ulcerative colitis: a novel therapeutic alternative. Am J Gastroenterol. 2000;95:1838–1839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

