Cost effectiveness of intraperitoneal compared with intravenous chemotherapy for women with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study
- PMID: 18757328
- PMCID: PMC2654369
- DOI: 10.1200/JCO.2007.13.1961
Cost effectiveness of intraperitoneal compared with intravenous chemotherapy for women with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study
Abstract
Purpose: To determine the cost effectiveness of intraperitoneal versus intravenous regimens for adjuvant treatment of optimally resected stage III ovarian cancer.
Patients and methods: A decision model was developed to compare the cost effectiveness at 7-, 11.5-, and 35-year horizons of intravenous carboplatin and paclitaxel (IV-CARBO/PAC), intravenous cisplatin and paclitaxel (IV-CIS/PAC), or intravenous paclitaxel followed by intraperitoneal cisplatin and paclitaxel (IP-CIS/PAC). Survival data were from women participating in representative Gynecologic Oncology Group (GOG) protocols. Medicare reimbursement rates and the Agency for Healthcare Research and Quality Database were used to estimate costs for treatment regimens and grade 3 to 4 adverse effects, respectively.
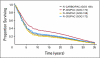
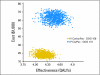
Results: Median predicted survival was 66, 57, 51, and 48 months for IP-CIS/PAC, IV-CARBO/PAC, IV-CIS/PAC (GOG 172), or IV-CIS/PAC (GOG 158), respectively. Across a range of analyses, IV-CIS/PAC was more costly and had lower life expectancy than IV-CARBO/PAC. Compared with IV-CARBO/PAC, IP-CIS/PAC had an incremental cost-effectiveness ratio (ICER) of $180,022 per quality-adjusted life year (QALY) saved at a 7-year time horizon, $71,835/QALY at 11.5 years, and $32,053/QALY over a lifetime. Extending the survival advantage of IP-CIS/PAC over 11.5 years and a lifetime results in ICERs of $26,249 and $23,973, respectively. Assuming IP-CIS/PAC and IV-CIS/PAC were equally effective when administered on an outpatient basis, the ICER of IP-CIS/PAC compared with IV-CARBO/PAC was $26,311.
Conclusion: Inpatient IP-CIS/PAC, while not cost effective compared with IV-CARBO/PAC at 7 years, becomes cost effective if a longer time horizon is modeled and/or a survival benefit can be assumed to persist longer than currently available data. Outpatient IP-CIS/PAC may also be cost effective compared with IV-CARBO/PAC if proven as effective as inpatient IP-CIS/PAC.
Figures

Similar articles
-
Intraperitoneal cisplatin and paclitaxel versus intravenous carboplatin and paclitaxel chemotherapy for Stage III ovarian cancer: a cost-effectiveness analysis.Gynecol Oncol. 2007 Sep;106(3):476-81. doi: 10.1016/j.ygyno.2007.05.043. Epub 2007 Aug 3. Gynecol Oncol. 2007. PMID: 17688927
-
Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study.J Clin Oncol. 2019 Jun 1;37(16):1380-1390. doi: 10.1200/JCO.18.01568. Epub 2019 Apr 19. J Clin Oncol. 2019. PMID: 31002578 Free PMC article. Clinical Trial.
-
Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group.J Clin Oncol. 2001 Feb 15;19(4):1001-7. doi: 10.1200/JCO.2001.19.4.1001. J Clin Oncol. 2001. PMID: 11181662 Clinical Trial.
-
Paclitaxel: a pharmacoeconomic review of its use in the treatment of ovarian cancer.Pharmacoeconomics. 2001;19(12):1227-59. doi: 10.2165/00019053-200119120-00005. Pharmacoeconomics. 2001. PMID: 11772158 Review.
-
Carboplatin versus cisplatin in ovarian cancer.Semin Oncol. 1995 Oct;22(5 Suppl 12):88-90. Semin Oncol. 1995. PMID: 7481869 Review.
Cited by
-
Comparison of methods to estimate health state utilities for ovarian cancer using quality of life data: a Gynecologic Oncology Group study.Gynecol Oncol. 2013 Feb;128(2):175-80. doi: 10.1016/j.ygyno.2012.10.024. Epub 2012 Oct 30. Gynecol Oncol. 2013. PMID: 23123576 Free PMC article.
-
Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment.Gynecol Oncol. 2009 May;113(2):216-20. doi: 10.1016/j.ygyno.2008.12.026. Epub 2009 Feb 12. Gynecol Oncol. 2009. PMID: 19217148 Free PMC article.
-
Cost Effectiveness of Chemotherapeutic Agents and Targeted Biologics in Ovarian Cancer: A Systematic Review.Pharmacoeconomics. 2015 Nov;33(11):1155-85. doi: 10.1007/s40273-015-0304-9. Pharmacoeconomics. 2015. PMID: 26072142
-
Consolidation paclitaxel is more cost-effective than bevacizumab following upfront treatment of advanced epithelial ovarian cancer.Gynecol Oncol. 2011 Sep;122(3):473-8. doi: 10.1016/j.ygyno.2011.05.014. Epub 2011 Jun 12. Gynecol Oncol. 2011. PMID: 21665250 Free PMC article.
-
Clinical utility of targeted treatments in the management of epithelial ovarian cancer.Biologics. 2012;6:233-44. doi: 10.2147/BTT.S29356. Epub 2012 Jul 26. Biologics. 2012. PMID: 22904615 Free PMC article.
References
-
- Alberts DS, Liu PY, Hannigan EV, et al: Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950-1955, 1996 - PubMed
-
- Markman M, Bundy BN, Alberts DS, et al: Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group J Clin Oncol 19:1001-1007, 2001 - PubMed
-
- Armstrong DK, Bundy B, Wenzel L, et al: Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34-43, 2006 - PubMed
-
- Wenzel LB, Huang H, Armstrong D, et al: Quality of live (QOL) results of a randomized study of intravenous (IV) paclitaxel and cisplatin vs IV paclitaxel, intraperitoneal (IP) cisplatin and IP paclitaxel in optimal stage III epithelial ovarian cancer: A Gynecologic Oncology Group trial. J Clin Oncol 22:454s, 2004. (abstr 5026)
-
- Ozols RF, Bookman MA, du Bois A, et al: Intraperitoneal cisplatin therapy in ovarian cancer: Comparison with standard intravenous carboplatin and paclitaxel. Gynecol Oncol 103:1-6, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

