Efficient cross-presentation depends on autophagy in tumor cells
- PMID: 18757401
- PMCID: PMC2905686
- DOI: 10.1158/0008-5472.CAN-08-0161
Efficient cross-presentation depends on autophagy in tumor cells
Abstract
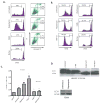
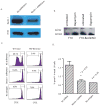
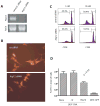
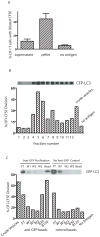
Cross-presentation of antigens is critical for the induction of adaptive immunity against tumor cells and infectious pathogens. Currently, it is not known how cross-presentation of tumor antigens is regulated by autophagy. Using both HEK 293T cells that expressed the model antigen OVA and melanoma cells as antigen donors, we show that macroautophagy in tumor cells is essential for cross-presentation by dendritic cells both in vitro and in vivo. Inhibition of autophagy abolished cross-presentation almost completely, whereas induction of autophagy dramatically enhanced the cross-presentation of tumor antigens. Moreover, purified autophagosomes were found to be efficient antigen carriers for cross-presentation. Our findings not only identified a novel role for autophagy as an active process in antigen sequestration and delivery to dendritic cells for cross-presentation, but also suggested, for the first time, that isolated autophagosomes may have potential as potent vaccines for immunotherapy against cancer and infectious diseases.
Figures
References
-
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. - PubMed
-
- Serna A, Ramirez MC, Soukhanova A, Sigal LJ. Cutting edge: efficient MHC class I cross-presentation during early vaccinia infection requires the transfer of proteasomal intermediates between antigen donor and presenting cells. J Immunol. 2003;171:5668–5672. - PubMed
-
- Norbury CC, Basta S, Donohue KB, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–1321. - PubMed
-
- Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

