Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells
- PMID: 18757424
- PMCID: PMC2562557
- DOI: 10.1158/0008-5472.CAN-08-0643
Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells
Abstract
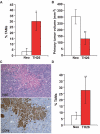
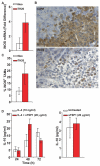
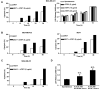
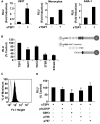
Inhibition of tumor growth by thrombospondin (TSP) 1 is generally attributed to its antiangiogenic activity, but effects on tumor immunity should also be considered. We show that overexpression of TSP1 in melanoma cells increases macrophage recruitment into xenograft tumors grown in nude or beige/nude mice. In vitro, TSP1 acutely induces expression of plasminogen activator inhibitor-1 (PAI-1) by monocytic cells, suggesting that TSP1-induced macrophage recruitment is at least partially mediated by PAI-1. Tumor-associated macrophages (TAM) can either promote or limit tumor progression. The percentage of M1-polarized macrophages expressing inducible nitric oxide synthase is increased in TSP1-expressing tumors. Furthermore, soluble TSP1 stimulates killing of breast carcinoma and melanoma cells by IFN-gamma-differentiated U937 cells in vitro via release of reactive oxygen species. TSP1 causes a significant increase in phorbol ester-mediated superoxide generation from differentiated monocytes by interaction with alpha(6)beta(1) integrin through its NH(2)-terminal region. The NH(2)-terminal domain of TSP2 also stimulates monocyte superoxide production. Extracellular calcium is required for the TSP1-induced macrophage respiratory burst. Thus, TSP1 may play an important role in antitumor immunity by enhancing recruitment and activation of M1 TAMs, which provides an additional selective pressure for loss of TSP1 and TSP2 expression during tumor progression.
Figures


Similar articles
-
The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice.Am J Pathol. 2002 Sep;161(3):831-9. doi: 10.1016/S0002-9440(10)64243-5. Am J Pathol. 2002. PMID: 12213711 Free PMC article.
-
Thrombospondin-1 triggers cell migration and development of advanced prostate tumors.Cancer Res. 2011 Dec 15;71(24):7649-58. doi: 10.1158/0008-5472.CAN-11-0833. Epub 2011 Oct 28. Cancer Res. 2011. PMID: 22037878
-
Endothelial-derived thrombospondin-1 promotes macrophage recruitment and apoptotic cell clearance.J Cell Mol Med. 2010 Jul;14(7):1922-34. doi: 10.1111/j.1582-4934.2009.00799.x. Epub 2009 Jun 5. J Cell Mol Med. 2010. PMID: 19508384 Free PMC article.
-
Thrombospondin-1 and cutaneous melanoma.J Cutan Med Surg. 2003 Mar-Apr;7(2):136-41. doi: 10.1007/s10227-002-0164-8. Epub 2002 Nov 27. J Cutan Med Surg. 2003. PMID: 12447621 Review.
-
Thrombospondin-1, PECAM-1, and regulation of angiogenesis.Histol Histopathol. 1999 Jan;14(1):285-94. doi: 10.14670/HH-14.285. Histol Histopathol. 1999. PMID: 9987673 Review.
Cited by
-
Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model.PLoS One. 2011;6(10):e26656. doi: 10.1371/journal.pone.0026656. Epub 2011 Oct 24. PLoS One. 2011. PMID: 22039525 Free PMC article.
-
M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma.J Exp Clin Cancer Res. 2018 Jul 9;37(1):143. doi: 10.1186/s13046-018-0815-2. J Exp Clin Cancer Res. 2018. PMID: 29986759 Free PMC article.
-
Signaling and stress: The redox landscape in NOS2 biology.Free Radic Biol Med. 2015 Oct;87:204-25. doi: 10.1016/j.freeradbiomed.2015.06.002. Epub 2015 Jun 24. Free Radic Biol Med. 2015. PMID: 26117324 Free PMC article. Review.
-
Comparative analysis of assays to measure CAR T-cell-mediated cytotoxicity.Nat Protoc. 2021 Mar;16(3):1331-1342. doi: 10.1038/s41596-020-00467-0. Epub 2021 Feb 15. Nat Protoc. 2021. PMID: 33589826 Free PMC article. Review.
-
High Serum miR-361-3p Predicts Early Postdischarge Infections after Autologous Stem Cell Transplantation.Infect Chemother. 2024 Sep;56(3):339-350. doi: 10.3947/ic.2024.0021. Epub 2024 Jun 4. Infect Chemother. 2024. PMID: 39098001 Free PMC article.
References
-
- Stenina OI, Byzova TV, Adams JC, McCarthy JJ, Topol EJ, Plow EF. Coronary artery disease and the thrombospondin single nucleotide polymorphisms. Int J Biochem Cell Biol. 2004;36:1013–30. - PubMed
-
- Crawford SE, Stellmach V, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–70. - PubMed
-
- Isenberg JS, Romeo MJ, Abu-Asab M, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–20. - PubMed
-
- Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. Faseb J. 1996;10:1183–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

