Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin
- PMID: 18757558
- PMCID: PMC2553619
- DOI: 10.1105/tpc.107.056812
Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin
Abstract
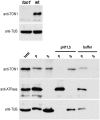
Plant cells have specific microtubule structures involved in cell division and elongation. The tonneau1 (ton1) mutant of Arabidopsis thaliana displays drastic defects in morphogenesis, positioning of division planes, and cellular organization. These are primarily caused by dysfunction of the cortical cytoskeleton and absence of the preprophase band of microtubules. Characterization of the ton1 insertional mutant reveals complex chromosomal rearrangements leading to simultaneous disruption of two highly similar genes in tandem, TON1a and TON1b. TON1 proteins are conserved in land plants and share sequence motifs with human centrosomal proteins. The TON1 protein associates with soluble and microsomal fractions of Arabidopsis cells, and a green fluorescent protein-TON1 fusion labels cortical cytoskeletal structures, including the preprophase band and the interphase cortical array. A yeast two-hybrid screen identified Arabidopsis centrin as a potential TON1 partner. This interaction was confirmed both in vitro and in plant cells. The similarity of TON1 with centrosomal proteins and its interaction with centrin, another key component of microtubule organizing centers, suggests that functions involved in the organization of microtubule arrays by the centrosome were conserved across the evolutionary divergence between plants and animals.
Figures






References
-
- Andersen, J.S., Wilkinson, C.J., Mayor, T., Mortensen, P., Nigg, E.A., and Mann, M. (2003). Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426 570–574. - PubMed
-
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (1998). Current Protocols in Molecular Biology. (New York: John Wiley & Sons).
-
- Ayaydin, F., Vissi, E., Meszaros, T., Miskolczi, P., Kovacs, I., Feher, A., Dombradi, V., Erdodi, F., Gergely, P., and Dudits, D. (2000). Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M transition and early mitotic microtubule organisation in alfalfa. Plant J. 23 85–96. - PubMed
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. III 316 1194–1199.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

