Multiplexed FRET to image multiple signaling events in live cells
- PMID: 18757561
- PMCID: PMC2576366
- DOI: 10.1529/biophysj.108.139204
Multiplexed FRET to image multiple signaling events in live cells
Abstract
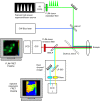
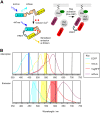
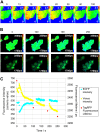
We report what to our knowledge is a novel approach for simultaneous imaging of two different Förster resonance energy transfer (FRET) sensors in the same cell with minimal spectral cross talk. Previous methods based on spectral ratiometric imaging of the two FRET sensors have been limited by the availability of suitably bright acceptors for the second FRET pair and the spectral cross talk incurred when measuring in four spectral windows. In contrast to spectral ratiometric imaging, fluorescence lifetime imaging (FLIM) requires measurement of the donor fluorescence only and is independent of emission from the acceptor. By combining FLIM-FRET of the novel red-shifted TagRFP/mPlum FRET pair with spectral ratiometric imaging of an ECFP/Venus pair we were thus able to maximize the spectral separation between our chosen fluorophores while at the same time overcoming the low quantum yield of the far red acceptor mPlum. Using this technique, we could read out a TagRFP/mPlum intermolecular FRET sensor for reporting on small Ras GTP-ase activation in live cells after epidermal growth factor stimulation and an ECFP/Venus Cameleon FRET sensor for monitoring calcium transients within the same cells. The combination of spectral ratiometric imaging of ECFP/Venus and high-speed FLIM-FRET of TagRFP/mPlum can thus increase the spectral bandwidth available and provide robust imaging of multiple FRET sensors within the same cell. Furthermore, since FLIM does not require equal stoichiometries of donor and acceptor, this approach can be used to report on both unimolecular FRET biosensors and protein-protein interactions with the same cell.
Figures
References
-
- Jares-Erijman, E. A., and T. M. Jovin. 2006. Imaging molecular interactions in living cells by FRET microscopy. Curr. Opin. Chem. Biol. 10:409–416. - PubMed
-
- Peyker, A., O. Rocks, and P. I. Bastiaens. 2005. Imaging activation of two Ras isoforms simultaneously in a single cell. ChemBioChem. 6:78–85. - PubMed
-
- Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. Giepmans, A. E. Palmer, and R. Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma. red fluorescent protein. Nat. Biotechnol. 22:1567–1572. - PubMed
-
- Piljic, A., and C. Schultz. 2008. Simultaneous recording of multiple cellular events by FRET. ACS Chem. Biol. 3:156–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

