TLRs and innate immunity
- PMID: 18757776
- PMCID: PMC2644070
- DOI: 10.1182/blood-2008-07-019307
TLRs and innate immunity
Abstract
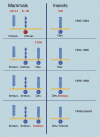
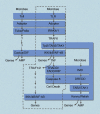
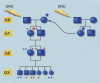
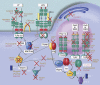
One of the most fundamental questions in immunology pertains to the recognition of non-self, which for the most part means microbes. How do we initially realize that we have been inoculated with microbes, and how is the immune response ignited? Genetic studies have made important inroads into this question during the past decade, and we now know that in mammals, a relatively small number of receptors operate to detect signature molecules that herald infection. One or more of these signature molecules are displayed by almost all microbes. These receptors and the signals they initiate have been studied in depth by random germline mutagenesis and positional cloning (forward genetics). Herein is a concise description of what has been learned about the Toll-like receptors, which play an essential part in the perception of microbes and shape the complex host responses that occur during infection.
Figures
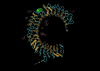
References
-
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology 20. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. - PubMed
-
- Pfeiffer R. Untersuchungen über das Choleragift. Z Hygiene. 1892;11:393–412.
-
- Shear MJ, Andervont HB. Chemical treatment of tumors. III. Separation of hemorrhage-producing fraction of B. coli filtrate. Proc Soc Exp Biol Med. 1936;34:323–325.
-
- Hartwell JL, Shear MJ, Adams JR., Jr Chemical treatment of tumors. VII. Nature of the hemorrhage-producing fraction from Serratia marcescens (Bacillus prodigiosus) culture filtrate. J Natl Cancer Inst. 1943;4:107–122.
-
- Kahler H, Shear MJ, Hartwell JL. Chemical treatment of tumors. VIII. Ultracentrifugal and electrophoretic analysis of the hemorrhage-producing fraction from Serratia marcescens (Bacillus prodigiosus) culture filtrate. J. Natl Cancer Inst. 1943;4:123–129.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

