Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis
- PMID: 18757836
- PMCID: PMC3837458
- DOI: 10.1194/jlr.R800018-JLR200
Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis
Abstract
Triacylglycerols (triglycerides) (TGs) are the major storage molecules of metabolic energy and FAs in most living organisms. Excessive accumulation of TGs, however, is associated with human diseases, such as obesity, diabetes mellitus, and steatohepatitis. The final and the only committed step in the biosynthesis of TGs is catalyzed by acyl-CoA:diacylglycerol acyltransferase (DGAT) enzymes. The genes encoding two DGAT enzymes, DGAT1 and DGAT2, were identified in the past decade, and the use of molecular tools, including mice deficient in either enzyme, has shed light on their functions. Although DGAT enzymes are involved in TG synthesis, they have distinct protein sequences and differ in their biochemical, cellular, and physiological functions. Both enzymes may be useful as therapeutic targets for diseases. Here we review the current knowledge of DGAT enzymes, focusing on new advances since the cloning of their genes, including possible roles in human health and diseases.
Figures

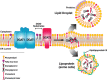

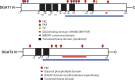
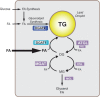
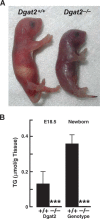
References
-
- Stryer L. Biochemistry. W.H. Freeman & Co.; New York, NY: 1988. p. 471.
-
- Unger RH. Lipotoxic diseases. Annu. Rev. Med. 2002;53:319–336. - PubMed
-
- Friedman J. Fat in all the wrong places. Nature. 2002;415:268–269. - PubMed
-
- Kennedy E. Metabolism of lipides. Annu. Rev. Biochem. 1957;26:119–148. - PubMed
-
- Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 1980;49:459–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

