Mechanisms of Ras membrane organization and signalling: Ras on a rocker
- PMID: 18758236
- PMCID: PMC2782589
- DOI: 10.4161/cc.7.17.6596
Mechanisms of Ras membrane organization and signalling: Ras on a rocker
Abstract
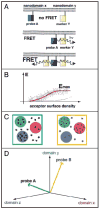
Understanding the signalling function of Ras GTPases has been the focus of much research for over 20 years. Both the catalytic domain and the membrane anchoring C terminal hypervariable region (HVR) of Ras are necessary for its cellular function. However, while the highly conserved catalytic domain has been characterized in atomic detail, the structure of the full-length membrane-bound Ras has remained elusive. Lack of structural knowledge on the full-length protein limited our understanding of Ras signalling. For example, structures of the Ras catalytic domain solved in complex with effectors do not provide a basis for the functional specificity of different Ras isoforms. Recent molecular dynamics simulations in combination with biophysical and cell biological experiments have shown that the HVR and parts of the G domain cofunction with the lipid tails to anchor H-ras to the plasma membrane. In the GTP-bound state, H-ras adopts an orientation that allows read out by Ras effectors and translation into corresponding MAPK signalling. Here we discuss details of an analysis that suggests a novel balance model for Ras functioning. The balance model rationalizes Ras membrane orientation and may help explain isoform specific interactions of Ras with its effectors and modulators.
Figures





References
-
- Abankwa D, Vogel H. A FRET map of membrane anchors suggests distinct microdomains of heterotrimeric G proteins. J Cell Sci. 2007;120:2953–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
