Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway
- PMID: 18758466
- PMCID: PMC3732485
- DOI: 10.1038/ni.1645
Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway
Abstract
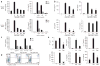
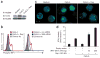
Robust production of type I interferon (IFN-alpha/beta) in plasmacytoid dendritic cells (pDCs) is crucial for antiviral immunity. Here we show involvement of the mammalian target of rapamycin (mTOR) pathway in regulating interferon production by pDCs. Inhibition of mTOR or its 'downstream' mediators, the p70 ribosomal S6 protein kinases p70S6K1 and p70S6K2, during pDC activation by Toll-like receptor 9 (TLR9) blocked the interaction of TLR9 with the adaptor MyD88 and subsequent activation of the interferon-regulatory factor IRF7, which resulted in impaired IFN-alpha/beta production. Microarray analysis confirmed that inhibition of mTOR by the immunosuppressive drug rapamycin suppressed antiviral and anti-inflammatory gene expression. Consistent with this, targeting rapamycin-encapsulated microparticles to antigen-presenting cells in vivo resulted in less IFN-alpha/beta production in response to CpG DNA or the yellow fever vaccine virus strain 17D. Thus, mTOR signaling is crucial in TLR-mediated IFN-alpha/beta responses by pDCs.
Figures




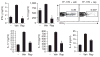
References
-
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. - PubMed
-
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. - PubMed
-
- Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. - PubMed
-
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. - PubMed
-
- Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005;26:221–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI048638/AI/NIAID NIH HHS/United States
- R01 DK057665/DK/NIDDK NIH HHS/United States
- AI0564499/AI/NIAID NIH HHS/United States
- R37 DK057665/DK/NIDDK NIH HHS/United States
- R01 AI048638/AI/NIAID NIH HHS/United States
- DK057665/DK/NIDDK NIH HHS/United States
- N01 AI050019/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- AI048638/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- AI05726601/AI/NIAID NIH HHS/United States
- AI057157/AI/NIAID NIH HHS/United States
- R56 AI048638/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

