Notch tumor suppressor function
- PMID: 18758480
- PMCID: PMC2747622
- DOI: 10.1038/onc.2008.225
Notch tumor suppressor function
Abstract
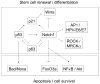
Cancer development results from deregulated control of stem cell populations and alterations in their surrounding environment. Notch signaling is an important form of direct cell-cell communication involved in cell fate determination, stem cell potential and lineage commitment. The biological function of this pathway is critically context dependent. Here we review the pro-differentiation role and tumor suppressing function of this pathway, as revealed by loss-of-function in keratinocytes and skin, downstream of p53 and in cross-connection with other determinants of stem cell potential and/or tumor formation, such as p63 and Rho/CDC42 effectors. The possibility that Notch signaling elicits a duality of signals, involved in growth/differentiation control and cell survival will be discussed, in the context of novel approaches for cancer therapy.
Figures

Similar articles
-
Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch.J Pathol. 2017 Aug;242(4):448-462. doi: 10.1002/path.4917. J Pathol. 2017. PMID: 28497579 Free PMC article.
-
The role of Notch signaling in human cervical cancer: implications for solid tumors.Oncogene. 2008 Sep 1;27(38):5110-4. doi: 10.1038/onc.2008.224. Oncogene. 2008. PMID: 18758479 Review.
-
Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases.Genes Dev. 2007 Mar 1;21(5):562-77. doi: 10.1101/gad.1484707. Genes Dev. 2007. PMID: 17344417 Free PMC article.
-
Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation.Genes Dev. 2006 Apr 15;20(8):1028-42. doi: 10.1101/gad.1406006. Genes Dev. 2006. PMID: 16618808 Free PMC article.
-
Notch signaling in the integrated control of keratinocyte growth/differentiation and tumor suppression.Semin Cancer Biol. 2004 Oct;14(5):374-86. doi: 10.1016/j.semcancer.2004.04.017. Semin Cancer Biol. 2004. PMID: 15288263 Review.
Cited by
-
The roles of Notch1 expression in the migration of intrahepatic cholangiocarcinoma.BMC Cancer. 2013 May 20;13:244. doi: 10.1186/1471-2407-13-244. BMC Cancer. 2013. PMID: 23688168 Free PMC article.
-
Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells.Proc Natl Acad Sci U S A. 2012 Dec 4;109(49):20035-40. doi: 10.1073/pnas.1213241109. Epub 2012 Nov 19. Proc Natl Acad Sci U S A. 2012. PMID: 23169653 Free PMC article.
-
A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration.Cell Biosci. 2019 Jan 5;9:7. doi: 10.1186/s13578-018-0264-9. eCollection 2019. Cell Biosci. 2019. PMID: 30627420 Free PMC article. Review.
-
ΔNp63α promotes cellular quiescence via induction and activation of Notch3.Cell Cycle. 2011 Sep 15;10(18):3111-8. doi: 10.4161/cc.10.18.17300. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21912215 Free PMC article.
-
An overview of notch signaling in adult tissue renewal and maintenance.Curr Alzheimer Res. 2012 Feb;9(2):227-40. doi: 10.2174/156720512799361600. Curr Alzheimer Res. 2012. PMID: 21605032 Free PMC article. Review.
References
-
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–93. - PubMed
-
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. - PubMed
-
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271:1826–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

