Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab
- PMID: 18759253
- PMCID: PMC2849723
- DOI: 10.1002/cncr.23824
Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab
Abstract
Background: Patients with chronic lymphocytic leukemia (CLL) usually are treated only for progressive disease. However, the discovery of biologic predictors of a high risk of disease progression, together with the development of newer, more targeted therapies, could change this paradigm. In this phase 2 study, the authors tested the safety and efficacy of early treatment for patients with high-risk CLL using alemtuzumab and rituximab.
Methods: Patients were eligible for treatment if they were 1) previously untreated, 2) had no National Cancer Institute-Working Group 1996 criteria for treatment, and 3) had at least 1 marker of high-risk disease 17p13-, 11q22-, or a combination of unmutated IgVH and CD38+/ZAP70+). Treatment consisted of subcutaneous alemtuzumab (initial dose escalation followed by 30 mg on Monday, Wednesday, and Friday for 4 weeks) and intravenous rituximab (375 mg/m(2) per week x4 doses). All patients received Pneumocystis pneumonia and herpes virus prophylaxis and were monitored for cytomegalovirus reactivation.
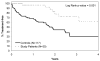
Results: Twenty-seven of 30 patients (90%) responded to therapy with 11 (37%) complete responses (CRs). Five patients (17%) patients who had a CR had no detectable minimal residual disease. The median response duration was 14.4 months, and only 9 patients required retreatment for progressive disease at the time of the current report (median follow-up, 17.6 months). Study patients had a significantly longer time from diagnosis to first treatment for CLL according to conventional indications than a comparison cohort with similar biologic risk profiles.
Conclusions: The therapy regimen used was safe and effective for early treatment of patients with high-risk CLL. Further studies will be required to determine whether this early treatment strategy decreases morbidity and mortality for high-risk CLL.
(c) 2008 American Cancer Society.
Figures




References
-
- Call TG, Phyliky RL, Noel P, Habermann TM, Beard CM, O’Fallon WM, et al. Incidence of chronic lymphocytic leukemia in Olmsted County, Minnesota, 1935 through 1989, with emphasis on changes in initial stage at diagnosis. Mayo Clin Proc. 1994;69:323–28. - PubMed
-
- Dighiero G, Binet J-L. When and how to treat chronic lymphocytic leukemia. N Engl J Med. 2000;343:1799–801. - PubMed
-
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–16. - PubMed
-
- Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

