A stereoselective synthesis of (+)-gonyautoxin 3
- PMID: 18759394
- PMCID: PMC2597710
- DOI: 10.1021/ja805651g
A stereoselective synthesis of (+)-gonyautoxin 3
Abstract
An asymmetric synthesis of the paralytic shellfish poison (PSP), (+)-gonyautoxin 3, is described. A unique, Rh-catalyzed amination reaction provides rapid access to the heteratom-rich, tricyclic core of the toxin, which is common to more than 30 related natural products. The completed route should facilitate the preparation of other naturally occurring PSPs and designed analogues thereof.
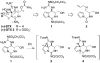
Figures


References
-
- Botana LM, editor. Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection. Marcel Dekker; New York: 2000.
-
-
For leading reviews, see: Llewellyn LE. Nat. Prod. Rep. 2006;23:200–222.. Hall S, Strichartz G, Moczydlowski E, Ravindran A, Reichardt PB. ACS Symp. Series. 1990;418:29–65.
-
-
-
Tanino H, Nakata T, Kaneko T, Kishi Y. J. Am. Chem. Soc. 1977;99:2818–2819.. Kishi Y. Heterocycles. 1980;14:1477–1495.. Jacobi PA, Martinelli MJ, Polanc S. J. Am. Chem. Soc. 1984;106:5594–5598.. Martinelli MJ, Brownstein AD, Jacobi PA, Polanc S. Croat. Chem. Acta. 1986;59:267–295.. Fleming JJ, Du Bois J. J. Am. Chem. Soc. 2006;128:3926–3927.. Fleming JJ, McReynolds MD, Du Bois J. J. Am. Chem. Soc. 2007;129:9964–9975.. Following their earlier report, Kishi et al. have described an asymmetric synthesis of (-)-decarbamoylsaxitoxin; see: Hong CY, Kishi Y. J. Am. Chem. Soc. 1992;114:7001–7006.
-
-
- Iwamoto O, Koshino H, Hashizume D, Nagasawa K. Angew. Chem., Int. Ed. 2007;46:8625–8628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

