Synthesis of high enantiopurity N-protected alpha-amino ketones by thiol ester-organostannane cross-coupling using pH-neutral conditions
- PMID: 18759432
- PMCID: PMC2654251
- DOI: 10.1021/ol8018456
Synthesis of high enantiopurity N-protected alpha-amino ketones by thiol ester-organostannane cross-coupling using pH-neutral conditions
Abstract
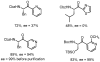
An efficient synthesis of high enantiopurity N-protected alpha-amino ketones is described. Complementing other studies using boronic acids and thiol esters, this Cu(I) diphenylphosphinate (CuDPP)-mediated, palladium-catalyzed coupling of alpha-amino thiol esters with aryl, heteroaryl, allyl, and alkenyl organostannanes gives N-protected alpha-amino ketones in high yields with high enantiopurity (in almost all cases) under mild and pH-neutral reaction conditions. The viability of pi-deficient heteroarylstannanes is an advantage of this reaction compared to the related boronic acid system.
Figures
Similar articles
-
Stereocontrolled synthesis of α-amino-α'-alkoxy ketones by a copper-catalyzed cross-coupling of peptidic thiol esters and α-alkoxyalkylstannanes.Org Lett. 2011 Jul 15;13(14):3682-5. doi: 10.1021/ol201330j. Epub 2011 Jun 15. Org Lett. 2011. PMID: 21675755 Free PMC article.
-
Ketone synthesis under neutral conditions. Cu(I) diphenylphosphinate-mediated, palladium-catalyzed coupling of thiol esters and organostannanes.Org Lett. 2003 Aug 21;5(17):3033-5. doi: 10.1021/ol034962x. Org Lett. 2003. PMID: 12916974
-
Ambient temperature synthesis of high enantiopurity N-protected peptidyl ketones by peptidyl thiol ester-boronic acid cross-coupling.J Am Chem Soc. 2007 Feb 7;129(5):1132-40. doi: 10.1021/ja0658719. J Am Chem Soc. 2007. PMID: 17263394 Free PMC article.
-
Palladium-catalyzed coupling of thiol esters with aryl and primary and secondary alkyl organoindium reagents.J Org Chem. 2005 Jun 10;70(12):4851-3. doi: 10.1021/jo050110u. J Org Chem. 2005. PMID: 15932328
-
Stereoselective anhydride openings.Chem Rev. 2007 Dec;107(12):5683-712. doi: 10.1021/cr068369f. Chem Rev. 2007. PMID: 18072807 Review. No abstract available.
Cited by
-
Synthetic Efforts toward the Synthesis of a Fluorinated Analog of 5-Aminolevulinic Acid: Practical Synthesis of Racemic and Enantiomerically Defined 3-Fluoro-5-aminolevulinic Acid.J Org Chem. 2024 Sep 6;89(17):12176-12186. doi: 10.1021/acs.joc.4c01070. Epub 2024 Aug 27. J Org Chem. 2024. PMID: 39189689 Free PMC article.
-
Nickel-Catalyzed Synthesis of Ketones from Alkyl Halides and Acid Chlorides: Preparation of Ethyl 4-Oxododecanoate.Organic Synth. 2016;93:50-62. doi: 10.15227/orgsyn.093.0050. Epub 2016 Feb 22. Organic Synth. 2016. PMID: 27667866 Free PMC article.
-
Stereocontrolled synthesis of α-amino-α'-alkoxy ketones by a copper-catalyzed cross-coupling of peptidic thiol esters and α-alkoxyalkylstannanes.Org Lett. 2011 Jul 15;13(14):3682-5. doi: 10.1021/ol201330j. Epub 2011 Jun 15. Org Lett. 2011. PMID: 21675755 Free PMC article.
-
Stereospecific Palladium-Catalyzed Acylation of Enantioenriched Alkylcarbastannatranes: A General Alternative to Asymmetric Enolate Reactions.Angew Chem Int Ed Engl. 2017 Jan 16;56(3):856-860. doi: 10.1002/anie.201609930. Epub 2016 Dec 16. Angew Chem Int Ed Engl. 2017. PMID: 27981696 Free PMC article.
-
On the Mechanism of Pd(0)-Catalyzed, Cu(I) Carboxylate-Mediated Thioorganic-Boronic Acid Desulfitative Coupling. A Non-innocent Role for Carboxylate Ligand.Organometallics. 2009 Jul 22;28(16):4639-4642. doi: 10.1021/om900602b. Organometallics. 2009. PMID: 20161122 Free PMC article.
References
-
- Timmons A, Seierstad M, Apodaca R, Epperson M, Pippel D, Brown S, Chang L, Scott B, Webb M, Chaplan SR, Breitenbucher JG. Bioorg. Med. Chem. Lett. 2008;18:2109–2113. - PubMed
- Maryanoff BE, Costanzo MJ. Bioorg. Med. Chem. 2008;16:1562–1596. - PubMed
- Costanzo MJ, Almond HR, Jr., Hecker LR, Schott MR, Yabut SC, Zhang H-C, Andrade-Gordon P, Corcoran TW, Giardino EC, Kauffman JA, Lewis JM, de Garavilla L, Haertlein BJ, Maryanoff BE. J. Med. Chem. 2005;48:1984–2008. - PubMed
- Douangamath A, Dale GE, D’Arcy A, Almstetter M, Eckl R, Frutos-Hoener A, Henkel B, Illgen K, Nerdinger S, Schulz H, MacSweeney A, Thormann M, Treml A, Pierau S, Wadman S, Oefner C. J. Med. Chem. 2004;47:1325–1328. - PubMed
- Costanzo MJ, Yabut SC, Almond HR, Jr., Andrade-Gordon P, Corcoran TW, de Garavilla L, Kauffman JA, Abraham WM, Recacha R, Chattopadhyay D, Maryanoff BE. J. Med. Chem. 2003;46:3865–3876. - PubMed
- Boger DL, Miyauchi H, Hedrick MP. Bioorg. Med. Chem. Lett. 2001;11:1517–1520. - PubMed
- Bachand B, Tarazi M, St-Denis Y, Edmunds JJ, Winocour PD, Leblond L, Siddiqui MA. Bioorg. Med. Chem. Lett. 2001;11:287–290. - PubMed
- Tripathy R, Ator MA, Mallamo JP. Bioorg. Med. Chem. Lett. 2000;10:2315–2319. - PubMed
- Marquis RW, Ru Y, Yamashita DS, Oh H-J, Yen J, Thompson SK, Carr TJ, Levy MA, Tomaszek TA, Ijames CF, Smith WW, Zhao B, Janson CA, Abdel-Meguid SS, D’Alessio KJ, McQueney MS, Veber DF. Bioorg. Med. Chem. 1999;7:581–588. - PubMed
- Calabretta R, Giordano C, Gallina C, Morea V, Consalvi V, Scandurra R. Eur. J. Med. Chem. 1995;30:931–941.
- Dragovich PS, Zhou R, Webber SE, Prins TJ, Kwok AK, Okano K, Fuhrman SA, Zalman LS, Maldonado FC, Brown EL, Meador JWI, Patick AK, Ford CE, Brothers MA, Binford SL, Matthews DA, Ferre RA, Worland ST. Bioorg. Med. Chem. Lett. 2000;10:45–48. - PubMed
- Eda M, Ashimori A, Akahoshi F, Yoshimura T, Inoue Y, Fukaya C, Nakajima M, Fukuyama H, Imada T, Nakamura N. Bioorg. Med. Chem. Lett. 1998;8:919–924. - PubMed
- Wagner BM, Smith RA, Coles PJ, Copp LJ, Ernest MJ, Krantz A. J. Med. Chem. 1994;37:1833–1840. - PubMed
- Mittag T, Christensen KL, Lindsay KB, Nielsen NC, Skrydstrup T. J. Org. Chem. 2008;73:1088–1092. - PubMed
-
- O’Donnell MJ, Drew MD, Pottorf RS, Scott WL. J. Comb. Chem. 2000;2:172–181. - PubMed
- Munoz B, Giam C-Z, Wong C-H. Bioorg. Med. Chem. Lett. 1994;2:1085–1090. - PubMed
- Fukuyama T, Tokuyama H. Aldrichimica Acta. 2004;37:87–96.
- Reetz MT. Angew. Chem., Int. Ed. Engl. 1991;30:1531–1546.
- Ooi T, Takeuchi M, Kato D, Uematsu Y, Tayama E, Sakai D, Maruoka K. J. Am. Chem. Soc. 2005;127:5073–5083. - PubMed
- Myers AG, Yoon T. Tetrahedron Lett. 1995;36:9429–9432.
- Florjancic AS, Sheppard GS. Synthesis. 2003:1653–1656.
- Klix RC, Chamberlin SA, Bhatia AV, Davis DA, Hayes TK, Rojas FG, Koops RW. Tetrahedron Lett. 1995;36:1791–1794.
- Paleo MR, Sardina FJ. Tetrahedron Lett. 1996;37:3403–3406.
- Vazquez J, Albericio F. Tetrahedron Lett. 2002;43:7499–7502.
- Katritzky AR, Le KNB, Khelashvili L, Mohapatra PP. J. Org. Chem. 2006;71:9861–9864. - PubMed
- Conrad K, Hsiao Y, Miller R. Tetrahedron Lett. 2005;46:8587–8589.
- Liu J, Ikemoto N, Petrillo D, Armstrong JD. Tetrahedron Lett. 2002;43:8223–8226.
- Bonini BF, Comes-Franchini M, Fochi M, Mazzanti G, Ricci A, Varchi G. Synlett. 1998:1013–1015.
- Sharma AK, Hergenrother PJ. Org. Lett. 2003;5:2107–2109. - PubMed
- Zhang Y, Rovis T. J. Am. Chem. Soc. 2004;126:15964–15965. - PubMed
-
- Urawa Y, Ogura K. Tetrahedron Lett. 2003;44:271–273.
- Haddach M, McCarthy JR. Tetrahedron Lett. 1999;40:3109–3112.
- Bumagin NA, Korolev DN. Tetrahedron Lett. 1999;40:3057–3060.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous