Protein secretion and outer membrane assembly in Alphaproteobacteria
- PMID: 18759741
- PMCID: PMC2635482
- DOI: 10.1111/j.1574-6976.2008.00130.x
Protein secretion and outer membrane assembly in Alphaproteobacteria
Abstract
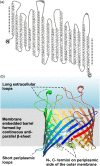
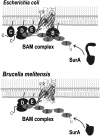
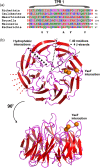
The assembly of beta-barrel proteins into membranes is a fundamental process that is essential in Gram-negative bacteria, mitochondria and plastids. Our understanding of the mechanism of beta-barrel assembly is progressing from studies carried out in Escherichia coli and Neisseria meningitidis. Comparative sequence analysis suggests that while many components mediating beta-barrel protein assembly are conserved in all groups of bacteria with outer membranes, some components are notably absent. The Alphaproteobacteria in particular seem prone to gene loss and show the presence or absence of specific components mediating the assembly of beta-barrels: some components of the pathway appear to be missing from whole groups of bacteria (e.g. Skp, YfgL and NlpB), other proteins are conserved but are missing characteristic domains (e.g. SurA). This comparative analysis is also revealing important structural signatures that are vague unless multiple members from a protein family are considered as a group (e.g. tetratricopeptide repeat (TPR) motifs in YfiO, beta-propeller signatures in YfgL). Given that the process of the beta-barrel assembly is conserved, analysis of outer membrane biogenesis in Alphaproteobacteria, the bacterial group that gave rise to mitochondria, also promises insight into the assembly of beta-barrel proteins in eukaryotes.
Figures

References
-
- Alcock FH, Grossmann JG, Gentle IE, Likić VA, Lithgow T, Tokatlidis K. Conserved substrate binding by chaperones in the bacterial periplasm and the mitochondrial intermembrane space. Biochem J. 2008;409:377–387. - PubMed
-
- Andersson SG, Kurland CG. Reductive evolution of resident genomes. Trends Microbiol. 1998;6:263–268. - PubMed
-
- Baars L, Ytterberg AJ, Drew D, Wagner S, Thilo C, van Wijk KJ, de Gier JW. Defining the role of the Escherichia coli chaperone SecB using comparative proteomics. J Biol Chem. 2006;281:10024–10034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

