Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation
- PMID: 18759972
- PMCID: PMC2556656
- DOI: 10.1186/1742-2094-5-37
Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation
Abstract
Background: Alzheimer's disease (AD) is characterized by extensive loss of neurons in the brain of AD patients. Intracellular accumulation of beta-amyloid peptide (Abeta) has also shown to occur in AD. Neuro-inflammation has been known to play a role in the pathogenesis of AD.
Methods: In this study, we investigated neuro-inflammation and amyloidogenesis and memory impairment following the systemic inflammation generated by lipopolysaccharide (LPS) using immunohistochemistry, ELISA, behavioral tests and Western blotting.
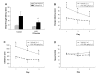
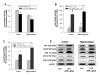
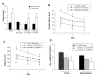
Results: Intraperitoneal injection of LPS, (250 microg/kg) induced memory impairment determined by passive avoidance and water maze tests in mice. Repeated injection of LPS (250 microg/kg, 3 or 7 times) resulted in an accumulation of Abeta1-42 in the hippocampus and cerebralcortex of mice brains through increased beta- and gamma-secretase activities accompanied with the increased expression of amyloid precursor protein (APP), 99-residue carboxy-terminal fragment of APP (C99) and generation of Abeta1-42 as well as activation of astrocytes in vivo. 3 weeks of pretreatment of sulindac sulfide (3.75 and 7.5 mg/kg, orally), an anti-inflammatory agent, suppressed the LPS-induced amyloidogenesis, memory dysfunction as well as neuronal cell death in vivo. Sulindac sulfide (12.5-50 microM) also suppressed LPS (1 microg/ml)-induced amyloidogenesis in cultured neurons and astrocytes in vitro.
Conclusion: This study suggests that neuro-inflammatory reaction could contribute to AD pathology, and anti-inflammatory agent could be useful for the prevention of AD.
Figures



Similar articles
-
Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models.J Neuroinflammation. 2012 Feb 19;9:35. doi: 10.1186/1742-2094-9-35. J Neuroinflammation. 2012. PMID: 22339795 Free PMC article.
-
Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties.J Nutr Biochem. 2013 Jan;24(1):298-310. doi: 10.1016/j.jnutbio.2012.06.011. Epub 2012 Sep 5. J Nutr Biochem. 2013. PMID: 22959056
-
Acceleration of amyloidogenesis and memory impairment by estrogen deficiency through NF-κB dependent beta-secretase activation in presenilin 2 mutant mice.Brain Behav Immun. 2016 Mar;53:113-122. doi: 10.1016/j.bbi.2015.11.013. Epub 2015 Nov 22. Brain Behav Immun. 2016. PMID: 26593275
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Neuroinflammatory processes in Alzheimer's disease.J Neural Transm (Vienna). 2010 Aug;117(8):919-47. doi: 10.1007/s00702-010-0438-z. Epub 2010 Jul 15. J Neural Transm (Vienna). 2010. PMID: 20632195 Review.
Cited by
-
Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memory impairments in a murine model of Alzheimer's disease.PLoS One. 2012;7(3):e33312. doi: 10.1371/journal.pone.0033312. Epub 2012 Mar 21. PLoS One. 2012. PMID: 22470449 Free PMC article.
-
Periodontitis and Alzheimer´s disease.Med Oral Patol Oral Cir Bucal. 2021 Jan 1;26(1):e43-e48. doi: 10.4317/medoral.23940. Med Oral Patol Oral Cir Bucal. 2021. PMID: 32701930 Free PMC article. Review.
-
Banhasasim-Tang Attenuates Lipopolysaccharide-Induced Cognitive Impairment by Suppressing Neuroinflammation in Mice.Nutrients. 2020 Jul 7;12(7):2019. doi: 10.3390/nu12072019. Nutrients. 2020. PMID: 32645984 Free PMC article.
-
Possible Mechanisms Involved in Attenuation of Lipopolysaccharide-Induced Memory Deficits by Methyl Jasmonate in Mice.Neurochem Res. 2016 Dec;41(12):3239-3249. doi: 10.1007/s11064-016-2050-6. Epub 2016 Sep 2. Neurochem Res. 2016. PMID: 27590498
-
NSAIDs may protect against age-related brain atrophy.Front Aging Neurosci. 2010 Sep 3;2:35. doi: 10.3389/fnagi.2010.00035. eCollection 2010. Front Aging Neurosci. 2010. PMID: 20877426 Free PMC article.
References
-
- Masliah E, LiCastro F. Neurodegenerative dementias: clinical features and pathological mechanisms. New York, McGraw-Hill; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

