Safety and efficacy of sertraline for depression in patients with CHF (SADHART-CHF): a randomized, double-blind, placebo-controlled trial of sertraline for major depression with congestive heart failure
- PMID: 18760123
- PMCID: PMC2659472
- DOI: 10.1016/j.ahj.2008.05.003
Safety and efficacy of sertraline for depression in patients with CHF (SADHART-CHF): a randomized, double-blind, placebo-controlled trial of sertraline for major depression with congestive heart failure
Abstract
Background: Sertraline, a selective serotonin-reuptake inhibitor, has demonstrated substantial mood improvement in patients with post myocardial infarction or with unstable angina. The impact of sertraline on the prognosis and depression of patients with chronic heart failure (HF) and comorbid major depressive disorder (MDD) is unknown.
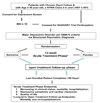
Method: This is a prospective, randomized, double-blind, placebo-controlled study designed to assess the safety and efficacy of sertraline in the treatment of MDD in patients with HF. The study is designed also to examine the effects of treating depression on cardiac events and morbidity/mortality in patients with HF. Approximately 500 men and women who are >or=45 years of age with current MDD and chronic systolic HF, characterized by left ventricular ejection fraction <or=45% and New York Heart Association class >or=II, comprise the study population. Eligible participants are randomized to either sertraline or placebo for a 12-week acute treatment phase. All patients, regardless of acute treatment phase completion, are followed routinely until the last subject completes 6-month follow-up. Quality of life and certain physiologic parameters, as well as pro-inflammatory and HF biomarkers, that may reflect the impact of sertraline in this particular population are measured at baseline and at the end of the acute treatment phase.
Conclusion: Because of the high prevalence of depression and its significant adverse impact on prognosis of patients with ischemic heart disease (IHD) and HF, the Safety and Efficacy of Sertraline for Depression in Patients with Chronic Heart Failure (SADHART-CHF) trial aims to assess the effects of sertraline on response of depression as well as on the cardiac prognosis of patients with HF.
Trial registration: ClinicalTrials.gov NCT00078286.
References
-
- Gradman A, Deedwania P, Cody R. Predictors of total mortality and sudden death in mild to moderate heart failure. JACC. 1989;14:564. - PubMed
-
- Cohn JN, Rector TS. Prognosis of congestive heart failure and predictors of mortality. Am J Cardiol. 1988;62:25A. - PubMed
-
- Braunwald E. Heart disease: a textbook of cardiovascular medicine. 4th edition. Vol. 11 W.B. Saunders Company; 1992.
-
- Sziachic J. Correlates and prognostic implications of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1986;55:1037. - PubMed
-
- Packer M, Gottieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with CHF. Am J Med. 1987;82 suppl. 3a:4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous