Estimating minimal clinically important differences of upper-extremity measures early after stroke
- PMID: 18760153
- PMCID: PMC2819021
- DOI: 10.1016/j.apmr.2008.02.022
Estimating minimal clinically important differences of upper-extremity measures early after stroke
Abstract
Objective: To estimate minimal clinically important difference (MCID) values of several upper-extremity measures early after stroke.
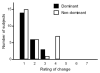
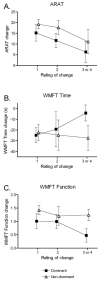
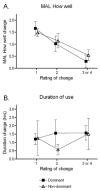
Design: Data in this report were collected during the Very Early Constraint-induced Therapy for Recovery of Stroke trial, an acute, single-blind randomized controlled trial of constraint-induced movement therapy. Subjects were tested at the prerandomization baseline assessment (average days poststroke, 9.5d) and the first posttreatment assessment (average days poststroke, 25.9d). At each time point, the affected upper extremity was evaluated with a battery of 6 tests. At the second assessment, subjects were also asked to provide a global rating of perceived changes in their affected upper extremity. Anchor-based MCID values were calculated separately for the affected dominant upper extremities and the affected nondominant upper extremities for each of the 6 tests.
Setting: Inpatient rehabilitation hospital.
Participants: Fifty-two people with hemiparesis poststroke.
Interventions: Not applicable.
Main outcome measures: Estimated MCID values for grip strength, composite upper-extremity strength, Action Research Arm Test (ARAT), Wolf Motor Function Test (WMFT), Motor Activity Log (MAL), and duration of upper-extremity use as measured with accelerometry.
Results: MCID values for grip strength were 5.0 and 6.2 kg for the affected dominant and nondominant sides, respectively. MCID values for the ARAT were 12 and 17 points, for the WMFT function score were 1.0 and 1.2 points, and for the MAL quality of movement score were 1.0 and 1.1 points for the 2 sides, respectively. MCID values were indeterminate for the dominant (composite strength), the nondominant (WMFT time score), and both affected sides (duration of use) for the other measures.
Conclusions: Our data provide some of the first estimates of MCID values for upper-extremity standardized measures early after stroke. Future studies with larger sample sizes are needed to refine these estimates and to determine whether MCID values are modified by time poststroke.
Conflict of interest statement
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Figures

References
-
- Finch E, Brooks D, Stratford PW, Mayo NE. Physical rehabilitation outcome measures. Hamilton, ON: BC Decker Inc; 2002.
-
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15. - PubMed
-
- Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (mcid): A literature review and directions for future research. Curr Opin Rheumatol. 2002;14:109–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

