A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus
- PMID: 18760319
- PMCID: PMC2747630
- DOI: 10.1016/j.vaccine.2008.07.103
A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus
Abstract
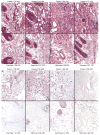
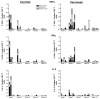
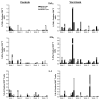
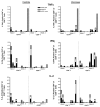
A previous study in nonhuman primates demonstrated that genetic immunization against the rhesus cytomegalovirus phosphoprotein 65-2 (pp65-2) and glycoprotein B (gB) antigens both stimulated antigen-specific antibodies and CD8 T cell responses, and significantly reduced plasma viral loads following intravenous challenge with RhCMV. It was also noted in this study that weak CD4 T cell and neutralizing antibody responses were generated by DNA alone. To broaden the type of immune responses, a DNA prime/protein boost strategy was used in seronegative macaques, consisting of four DNA immunizations against pp65-2, gB, and immediate-early 1 (IE1), followed by two boosts with formalin-inactivated RhCMV virions. This heterologous prime/boost strategy elicited robust antigen-specific CD4 and CD8 T cell responses in addition to biologically relevant neutralizing antibody titers. Animals were challenged with RhCMV delivered into four sites via a subcutaneous route. Skin biopsies of one of the inoculation sites 7 days post challenge revealed marked differences in the level of RhCMV replication between the vaccinated and control monkeys. Whereas the inoculation site in the controls was noted for a prominent inflammatory response and numerous cytomegalic, antigen-positive (IE1) cells, the inoculation site in the vaccinees was characterized by an absence of inflammation and antigen-positive cells. All five vaccinees developed robust recall responses to viral antigens, and four of them exhibited long-term viral immune responses consistent with effective control of viral expression and replication. These results demonstrate that a heterologous DNA prime/protein boost strategy greatly expands the breadth of antiviral immune responses and greatly reduces the level of viral replication at the primary site of challenge infection.
Figures


Similar articles
-
Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques.J Virol. 2011 Mar;85(6):2878-90. doi: 10.1128/JVI.00883-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191005 Free PMC article.
-
Immunogenicity and protective efficacy of DNA vaccines expressing rhesus cytomegalovirus glycoprotein B, phosphoprotein 65-2, and viral interleukin-10 in rhesus macaques.J Virol. 2007 Feb;81(3):1095-109. doi: 10.1128/JVI.01708-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108040 Free PMC article.
-
Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83.Viral Immunol. 2000;13(2):155-67. doi: 10.1089/vim.2000.13.155. Viral Immunol. 2000. PMID: 10892996
-
The next generation recombinant human cytomegalovirus vaccine candidates-beyond gB.Vaccine. 2012 Nov 19;30(49):6980-90. doi: 10.1016/j.vaccine.2012.09.056. Epub 2012 Oct 3. Vaccine. 2012. PMID: 23041121 Review.
-
Comparison of vaccine strategies against congenital CMV infection in the guinea pig model.J Clin Virol. 2008 Mar;41(3):224-30. doi: 10.1016/j.jcv.2007.10.008. Epub 2007 Dec 3. J Clin Virol. 2008. PMID: 18060834 Review.
Cited by
-
SIV antigen immunization induces transient antigen-specific T cell responses and selectively activates viral replication in draining lymph nodes in retroviral suppressed rhesus macaques.Retrovirology. 2011 Jul 13;8:57. doi: 10.1186/1742-4690-8-57. Retrovirology. 2011. PMID: 21752277 Free PMC article.
-
VennVax, a DNA-prime, peptide-boost multi-T-cell epitope poxvirus vaccine, induces protective immunity against vaccinia infection by T cell response alone.Vaccine. 2011 Jan 10;29(3):501-11. doi: 10.1016/j.vaccine.2010.10.064. Epub 2010 Nov 4. Vaccine. 2011. PMID: 21055490 Free PMC article.
-
A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques.J Virol. 2013 Feb;87(3):1322-32. doi: 10.1128/JVI.01669-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152525 Free PMC article.
-
Horizontal Transmission of Cytomegalovirus in a Rhesus Model Despite High-Level, Vaccine-Elicited Neutralizing Antibody and T-Cell Responses.J Infect Dis. 2022 Sep 4;226(4):585-594. doi: 10.1093/infdis/jiac129. J Infect Dis. 2022. PMID: 35413121 Free PMC article.
-
Human cytomegalovirus UL18 prevents priming of MHC-E- and MHC-II-restricted CD8+ T cells.Sci Immunol. 2024 Oct 11;9(100):eadp5216. doi: 10.1126/sciimmunol.adp5216. Epub 2024 Oct 11. Sci Immunol. 2024. PMID: 39392895 Free PMC article.
References
-
- Khanna R, Diamond DJ. Human cytomegalovirus vaccine: time to look for alternative options. Trends Mol Med. 2006 January;12(1):26–33. - PubMed
-
- Plotkin SA. Is there a formula for an effective CMV vaccine? J Clin Virol. 2002 August;25(Suppl 2):S13–21. - PubMed
-
- Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st century: a tool for decision making. Washington, D.C.: National Academy Press; 2000. - PubMed
-
- Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003 December;188(12):1868–74. - PubMed
-
- Schleiss MR, Bourne N, Jensen NJ, Bravo F, Bernstein DI. Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Viral Immunol. 2000;13(2):155–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

