NeuroD1 and Mash1 temporally regulate GnRH receptor gene expression in immortalized mouse gonadotrope cells
- PMID: 18760324
- PMCID: PMC2640340
- DOI: 10.1016/j.mce.2008.07.017
NeuroD1 and Mash1 temporally regulate GnRH receptor gene expression in immortalized mouse gonadotrope cells
Abstract
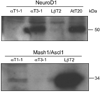
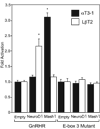
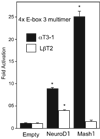
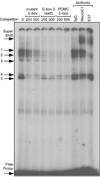
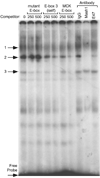
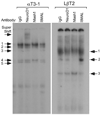
Accurate spatial and temporal expression of gonadotrope-specific genes, such as the gonadotropin-releasing hormone receptor (GnRHR) gene, is critical for gonadotrope maturation. Herein, we show that a specific E-box in the mouse GnRHR promoter binds two group A basic-helix-loop-helix (bHLH) transcription factors. Mutation of this E-box decreases expression in mouse gonadotrope-derived alphaT3-1 and LbetaT2 cell lines. Microarray and western blots show that the bHLH transcription factor NeuroD1 is strongly expressed in the gonadotrope progenitor, alphaT3-1, whereas Mash1 is strongly expressed in the more mature gonadotrope, LbetaT2. Over-expression of NeuroD1 or Mash1 increases expression of the GnRHR gene or a multimer of the E-box and this increase is lost upon mutation of the E-box. Electrophoretic mobility shift assays reveal that the GnRHR E-box binds NeuroD1 from alphaT3-1 cells, but binds Mash1 from LbetaT2 cells. The sequential binding of different members of the group A bHLH transcription factor family to mouse GnRHR E-box 3 as the gonadotrope differentiates may represent a mechanism necessary for proper spatial and temporal expression of the GnRHR during gonadotrope development.
Figures

Similar articles
-
Msx1 homeodomain protein represses the αGSU and GnRH receptor genes during gonadotrope development.Mol Endocrinol. 2013 Mar;27(3):422-36. doi: 10.1210/me.2012-1289. Epub 2013 Jan 31. Mol Endocrinol. 2013. PMID: 23371388 Free PMC article.
-
E-box regulation of gonadotropin-releasing hormone (GnRH) receptor expression in immortalized gonadotrope cells.Mol Cell Endocrinol. 2007 Nov 15;278(1-2):36-43. doi: 10.1016/j.mce.2007.08.008. Epub 2007 Aug 30. Mol Cell Endocrinol. 2007. PMID: 17928134
-
Activity of the porcine gonadotropin-releasing hormone receptor gene promoter is partially conferred by a distal gonadotrope specific element (GSE) within an upstream enhancing region, two proximal GSEs and a retinoid X receptor binding site.Reprod Biol Endocrinol. 2015 May 17;13:45. doi: 10.1186/s12958-015-0033-0. Reprod Biol Endocrinol. 2015. PMID: 25981521 Free PMC article.
-
[An ambiguous role of steroidogenic factor 1 in the rat GnRH receptor gene expression. Lessons from transgenic mice].J Soc Biol. 2004;198(1):73-9. J Soc Biol. 2004. PMID: 15146959 Review. French.
-
Targeting mediators of Wnt signalling pathways by GnRH in gonadotropes.J Mol Endocrinol. 2010 Apr;44(4):195-201. doi: 10.1677/JME-09-0168. Epub 2010 Feb 4. J Mol Endocrinol. 2010. PMID: 20133385 Review.
Cited by
-
Mechanisms underlying the tissue-specific and regulated activity of the Gnrhr promoter in mammals.Front Endocrinol (Lausanne). 2012 Dec 13;3:162. doi: 10.3389/fendo.2012.00162. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 23248618 Free PMC article.
-
Transcriptomic and Chromatin Landscape Analysis Reveals That Involvement of Pituitary Level Transcription Factors Modulate Incubation Behaviors of Magang Geese.Genes (Basel). 2023 Mar 28;14(4):815. doi: 10.3390/genes14040815. Genes (Basel). 2023. PMID: 37107573 Free PMC article.
-
Homeodomain Proteins SIX3 and SIX6 Regulate Gonadotrope-specific Genes During Pituitary Development.Mol Endocrinol. 2015 Jun;29(6):842-55. doi: 10.1210/me.2014-1279. Epub 2015 Apr 27. Mol Endocrinol. 2015. PMID: 25915183 Free PMC article.
-
Msx1 homeodomain protein represses the αGSU and GnRH receptor genes during gonadotrope development.Mol Endocrinol. 2013 Mar;27(3):422-36. doi: 10.1210/me.2012-1289. Epub 2013 Jan 31. Mol Endocrinol. 2013. PMID: 23371388 Free PMC article.
-
Pituitary gland development and disease: from stem cell to hormone production.Curr Top Dev Biol. 2013;106:1-47. doi: 10.1016/B978-0-12-416021-7.00001-8. Curr Top Dev Biol. 2013. PMID: 24290346 Free PMC article. Review.
References
-
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. - PubMed
-
- Albarracin CT, Kaiser UB, Chin WW. Isolation and characterization of the 5'-flanking region of the mouse gonadotropin-releasing hormone receptor gene. Endocrinology. 1994;135:2300–2306. - PubMed
-
- Duval DL, Nelson SE, Clay CM. The tripartite basal enhancer of the gonadotropin-releasing hormone (GnRH) receptor gene promoter regulates cell-specific expression through a novel GnRH receptor activating sequence. Mol. Endocrinol. 1997;11:1814–1821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

