A positive feedback signal transduction loop determines timing of cerebellar long-term depression
- PMID: 18760697
- PMCID: PMC2605966
- DOI: 10.1016/j.neuron.2008.06.026
A positive feedback signal transduction loop determines timing of cerebellar long-term depression
Abstract
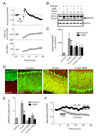
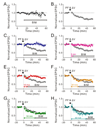
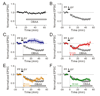
Synaptic activity produces short-lived second messengers that ultimately yield a long-term depression (LTD) of cerebellar Purkinje cells. Here, we test the hypothesis that these brief second messenger signals are translated into long-lasting biochemical signals by a positive feedback loop that includes protein kinase C (PKC) and mitogen-activated protein kinase. Histochemical "epistasis" experiments demonstrate the reciprocal activation of these kinases, and physiological experiments--including the use of a light-activated protein kinase--demonstrate that such reciprocal activation is required for LTD. Timed application of enzyme inhibitors reveals that this positive feedback loop causes PKC to be active for more than 20 min, allowing sufficient time for LTD expression. Such regenerative mechanisms may sustain other long-lasting forms of synaptic plasticity and could be a general mechanism for prolonging signal transduction networks.
Figures






References
-
- Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. - PubMed
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. - PubMed
-
- Anderson WW, Collingridge GL. The LTP program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods. 2001;108:71–83. - PubMed
-
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
-
- Atkins CM, Davare MA, Oh MC, Derkach V, Soderling TR. Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation. J. Neurosci. 2005;25:5604–5610. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

