Pax2/8 act redundantly to specify glycinergic and GABAergic fates of multiple spinal interneurons
- PMID: 18761336
- PMCID: PMC2849013
- DOI: 10.1016/j.ydbio.2008.08.009
Pax2/8 act redundantly to specify glycinergic and GABAergic fates of multiple spinal interneurons
Abstract
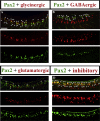
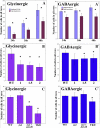
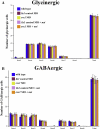
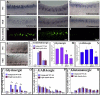
The spinal cord contains several distinct classes of neurons but it is still unclear how many of the functional characteristics of these cells are specified. One of the most crucial functional characteristics of a neuron is its neurotransmitter fate. In this paper, we show that in zebrafish most glycinergic and many GABAergic spinal interneurons express Pax2a, Pax2b and Pax8 and that these transcription factors are redundantly required for the neurotransmitter fates of many of these cells. We also demonstrate that the function of these Pax2/8 transcription factors is very specific: in embryos in which Pax2a, Pax2b and Pax8 are simultaneously knocked-down, many neurons lose their glycinergic and/or GABAergic characteristics, but they do not become glutamatergic or cholinergic and their soma morphologies and axon trajectories are unchanged. In mouse, Pax2 is required for correct specification of GABAergic interneurons in the dorsal horn, but it is not required for the neurotransmitter fates of other Pax2-expressing spinal neurons. Our results suggest that this is probably due to redundancy with Pax8 and that the function of Pax2/8 in specifying GABAergic and glycinergic neuronal fates is much broader than was previously appreciated and is highly conserved between different vertebrates.
Figures







Similar articles
-
Evx1 and Evx2 specify excitatory neurotransmitter fates and suppress inhibitory fates through a Pax2-independent mechanism.Neural Dev. 2016 Feb 19;11:5. doi: 10.1186/s13064-016-0059-9. Neural Dev. 2016. PMID: 26896392 Free PMC article.
-
Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord.Development. 2007 Jan;134(2):357-66. doi: 10.1242/dev.02717. Epub 2006 Dec 13. Development. 2007. PMID: 17166926
-
Lmx1b is required for the glutamatergic fates of a subset of spinal cord neurons.Neural Dev. 2016 Aug 23;11(1):16. doi: 10.1186/s13064-016-0070-1. Neural Dev. 2016. PMID: 27553035 Free PMC article.
-
Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord.Int J Mol Sci. 2022 Jan 13;23(2):834. doi: 10.3390/ijms23020834. Int J Mol Sci. 2022. PMID: 35055019 Free PMC article. Review.
-
GABAergic and glycinergic interneuron expression during spinal cord development: dynamic interplay between inhibition and excitation in the control of ventral network outputs.Prog Neurobiol. 2009 Sep;89(1):46-60. doi: 10.1016/j.pneurobio.2009.06.001. Epub 2009 Jun 17. Prog Neurobiol. 2009. PMID: 19539686 Review.
Cited by
-
Tal1, Gata2a, and Gata3 Have Distinct Functions in the Development of V2b and Cerebrospinal Fluid-Contacting KA Spinal Neurons.Front Neurosci. 2018 Mar 29;12:170. doi: 10.3389/fnins.2018.00170. eCollection 2018. Front Neurosci. 2018. PMID: 29651232 Free PMC article.
-
Evx1 and Evx2 specify excitatory neurotransmitter fates and suppress inhibitory fates through a Pax2-independent mechanism.Neural Dev. 2016 Feb 19;11:5. doi: 10.1186/s13064-016-0059-9. Neural Dev. 2016. PMID: 26896392 Free PMC article.
-
Maternal thyroid hormones are essential for neural development in zebrafish.Mol Endocrinol. 2014 Jul;28(7):1136-49. doi: 10.1210/me.2014-1032. Epub 2014 May 30. Mol Endocrinol. 2014. PMID: 24877564 Free PMC article.
-
Transcriptomics reveal an integrative role for maternal thyroid hormones during zebrafish embryogenesis.Sci Rep. 2017 Nov 30;7(1):16657. doi: 10.1038/s41598-017-16951-9. Sci Rep. 2017. PMID: 29192226 Free PMC article.
-
Transcriptional Regulators with Broad Expression in the Zebrafish Spinal Cord.bioRxiv [Preprint]. 2024 May 19:2024.02.14.580357. doi: 10.1101/2024.02.14.580357. bioRxiv. 2024. Update in: Dev Dyn. 2024 Nov;253(11):1036-1055. doi: 10.1002/dvdy.717. PMID: 38405913 Free PMC article. Updated. Preprint.
References
-
- Abramoff M.D., Magelhaes P.J., Ram S.J. Image Processing with Image J. Biophoton. Int. 2004;11:36–42.
-
- Ashworth R., Bolsover S.R. Spontaneous activity-independent intracellular calcium signals in the developing spinal cord of the zebrafish embryo. Brain Res. Dev. Brain Res. 2002;139:131–137. - PubMed
-
- Bernhardt R.R., Chitnis A.B., Lindamer L., Kuwada J.Y. Identification of spinal neurons in the embryonic and larval zebrafish. J. Comp. Neurol. 1990;302:603–616. - PubMed
-
- Borodinsky L.N., Root C.M., Cronin J.A., Sann S.B., Gu X., Spitzer N.C. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

