Development of recombinant varicella-zoster viruses expressing luciferase fusion proteins for live in vivo imaging in human skin and dorsal root ganglia xenografts
- PMID: 18761377
- PMCID: PMC2657092
- DOI: 10.1016/j.jviromet.2008.07.033
Development of recombinant varicella-zoster viruses expressing luciferase fusion proteins for live in vivo imaging in human skin and dorsal root ganglia xenografts
Abstract
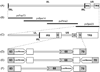
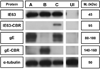
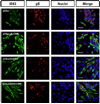
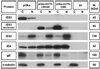
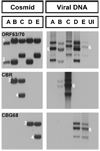
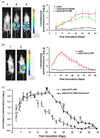
Varicella-zoster virus (VZV) is a host specific human pathogen that has been studied using human xenografts in SCID mice. Live whole-animal imaging is an emerging technique to measure protein expression in vivo using luminescence. Currently, it has only been possible to determine VZV protein expression in xenografts postmortem. Therefore, to measure immediate early (IE63) and late (glycoprotein E [gE]) protein expression in vivo viruses expressing IE63 or gE as luciferase fusion proteins were generated. Viable recombinant viruses pOka-63-luciferase and pOka-63/70-luciferase, which had luciferase genes fused to ORF63 and its duplicate ORF70, or pOka-gE-CBR were recovered that expressed IE63 or gE as fusion proteins and generated luminescent plaques. In contrast to pOka-63/70-luciferase viruses, the luciferase gene was rapidly lost in vitro when fused to a single copy of ORF63 or ORF68. IE63 expression was successfully measured in human skin and dorsal root ganglia xenografts infected with the genomically stable pOka-63/70-luciferase viruses. The progress of VZV infection in dorsal root ganglia xenografts was delayed in valacyclovir treated mice but followed a similar trend in untreated mice when the antiviral was withdrawn 28 days post-inoculation. Thus, IE63-luciferase fusion proteins were effective for investigating VZV infection and antiviral activity in human xenografts.
Figures

References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with Image. J. Bio-photon. Int. 2004;11:36–42.
-
- Baiker A, Bagowski C, Ito H, Sommer M, Zerboni L, Fabel K, Hay J, Ruyechan W, Arvin AM. The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J. Virol. 2004;78:1181–1194. - PMC - PubMed
-
- Besser J, Sommer MH, Zerboni L, Bagowski CP, Ito H, Moffat J, Ku CC, Arvin AM. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J. Virol. 2003;77:5964–5974. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

