Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype
- PMID: 18762025
- PMCID: PMC2613643
- DOI: 10.1016/j.cmet.2008.07.006
Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype
Retraction in
-
Retraction Notice to: Activation of the PPAR/PGC-1α Pathway Prevents a Bioenergetic Deficit and Effectively Improves a Mitochondrial Myopathy Phenotype.Cell Metab. 2016 Dec 13;24(6):889. doi: 10.1016/j.cmet.2016.11.006. Cell Metab. 2016. PMID: 27974182 Free PMC article. No abstract available.
Abstract
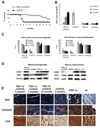
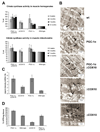
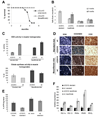
Neuromuscular disorders with defects in the mitochondrial ATP-generating system affect a large number of children and adults worldwide, but remain without treatment. We used a mouse model of mitochondrial myopathy, caused by a cytochrome c oxidase deficiency, to evaluate the effect of induced mitochondrial biogenesis on the course of the disease. Mitochondrial biogenesis was induced either by transgenic expression of peroxisome proliferator-activated receptor gamma (PPARgamma) coactivator alpha (PGC-1alpha) in skeletal muscle or by administration of bezafibrate, a PPAR panagonist. Both strategies successfully stimulated residual respiratory capacity in muscle tissue. Mitochondrial proliferation resulted in an enhanced OXPHOS capacity per muscle mass. As a consequence, ATP levels were conserved resulting in a delayed onset of the myopathy and a markedly prolonged life span. Thus, induction of mitochondrial biogenesis through pharmacological or metabolic modulation of the PPAR/PGC-1alpha pathway promises to be an effective therapeutic approach for mitochondrial disorders.
Figures

Similar articles
-
Endurance exercise is protective for mice with mitochondrial myopathy.J Appl Physiol (1985). 2009 May;106(5):1712-9. doi: 10.1152/japplphysiol.91571.2008. Epub 2009 Mar 12. J Appl Physiol (1985). 2009. Retraction in: J Appl Physiol (1985). 2016 Dec 1;121(6):1381. doi: 10.1152/japplphysiol.zdg-2008-retr.2016. PMID: 19286571 Free PMC article. Retracted.
-
alpha-Lipoic acid increases energy expenditure by enhancing adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling in the skeletal muscle of aged mice.Metabolism. 2010 Jul;59(7):967-76. doi: 10.1016/j.metabol.2009.10.018. Epub 2009 Dec 16. Metabolism. 2010. PMID: 20015518 Free PMC article.
-
Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington's disease.Hum Mol Genet. 2012 Mar 1;21(5):1124-37. doi: 10.1093/hmg/ddr541. Epub 2011 Nov 17. Hum Mol Genet. 2012. PMID: 22095692 Free PMC article.
-
Turn up the power - pharmacological activation of mitochondrial biogenesis in mouse models.Br J Pharmacol. 2014 Apr;171(8):1818-36. doi: 10.1111/bph.12413. Br J Pharmacol. 2014. PMID: 24102298 Free PMC article. Review.
-
PGC-1α, mitochondrial dysfunction, and Huntington's disease.Free Radic Biol Med. 2013 Sep;62:37-46. doi: 10.1016/j.freeradbiomed.2013.04.016. Epub 2013 Apr 19. Free Radic Biol Med. 2013. PMID: 23602910 Free PMC article. Review.
Cited by
-
Tissue-specific splicing of an Ndufs6 gene-trap insertion generates a mitochondrial complex I deficiency-specific cardiomyopathy.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6165-70. doi: 10.1073/pnas.1113987109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474353 Free PMC article.
-
Mitochondrial Diseases Part I: mouse models of OXPHOS deficiencies caused by defects in respiratory complex subunits or assembly factors.Mitochondrion. 2015 Mar;21:76-91. doi: 10.1016/j.mito.2015.01.009. Epub 2015 Feb 4. Mitochondrion. 2015. PMID: 25660179 Free PMC article. Review.
-
Emerging aspects of treatment in mitochondrial disorders.J Inherit Metab Dis. 2015 Jul;38(4):641-53. doi: 10.1007/s10545-015-9855-3. Epub 2015 May 12. J Inherit Metab Dis. 2015. PMID: 25962587 Review.
-
Context-Dependent Role of Mitochondrial Fusion-Fission in Clonal Expansion of mtDNA Mutations.PLoS Comput Biol. 2015 May 21;11(5):e1004183. doi: 10.1371/journal.pcbi.1004183. eCollection 2015 May. PLoS Comput Biol. 2015. PMID: 25996936 Free PMC article.
-
Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology.Semin Immunopathol. 2014 Jan;36(1):27-53. doi: 10.1007/s00281-013-0406-4. Epub 2013 Nov 21. Semin Immunopathol. 2014. PMID: 24258516 Review.
References
-
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. - PubMed
-
- Bastin J, Aubey F, Rotig A, Munnich A, Djouadi F. Activation of peroxisome proliferator activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients' cells lacking its components. J Clin Endocrinol Metab. 2008 - PubMed
-
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPAR{gamma}coactivator-1{alpha} improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

