The ontogeny of the porcine immune system
- PMID: 18762210
- PMCID: PMC7103207
- DOI: 10.1016/j.dci.2008.07.011
The ontogeny of the porcine immune system
Abstract
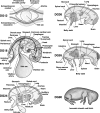
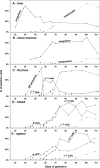
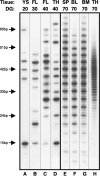
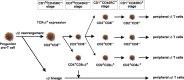
Cellular and humoral aspects of the immune response develop sequentially in the fetus. During the ontogeny, the pluripotent stem cells emerge and differentiate into all hematopoietic lineages. Basic questions including the identification of the first lympho-hematopoietic sites, the origin of T and B lymphocytes, the development of different subpopulations of alphabeta T, gammadelta T and B lymphocytes as well as development of innate immunity and the acquisition of full immunological capacities are discussed here for swine and compared with other species. The description of related topics such as fertilization, morphogenesis, maternal-fetal-neonatal physiology and early neonatal development are also discussed.
Figures



References
-
- Marrable A.W. Pitman Medical Publishing; Great Britain: 1971. The embryonic pig: a chronological account.
-
- Sterzl J., Silverstein A.M. Developmental aspects of immunity. Adv Immunol. 1967;6(1):337–371. - PubMed
-
- Butler J.E., Brown W.R. The immunoglobulins and immunoglobulin genes of swine. Vet Immunol Immunopathol. 1994;43(1-3):5–12. - PubMed
-
- Tlaskalova-Hogenova H., Mandel L., Trebichavsky I., Kovaru F., BArot R., Sterzl J. Development of immune responses in early pig ontogeny. Vet Immunol Immunopathol. 1994;43(1-3):135–142. - PubMed
-
- Butler J.E., Sun J., Weber P., Ford S.P., Rehakova Z., Sinkora J. Antibody repertoire development in fetal and neonatal piglets. IV. Switch recombination, primarily in fetal thymus occurs independent of environmental antigen and is only weakly associated with repertoire diversification. J Immunol. 2001;167(6):3239–3249. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

