Real-time quantitative PCR and fast QPCR have similar sensitivity and accuracy with HIV cDNA late reverse transcripts and 2-LTR circles
- PMID: 18762215
- PMCID: PMC2582487
- DOI: 10.1016/j.jviromet.2008.07.032
Real-time quantitative PCR and fast QPCR have similar sensitivity and accuracy with HIV cDNA late reverse transcripts and 2-LTR circles
Abstract
Real-time fluorescent quantitative PCR (universal QPCR) methods are used routinely in both academic and clinical research to measure HIV cDNA. Fast QPCR allows for faster ramping times between cycles and smaller reaction volumes, but may lose sensitivity and accuracy. We demonstrate that primer sets for HIV late reverse transcripts and 2-LTR circles have similar sensitivity and accuracy with either universal or fast QPCR methods. However, both cost and time are reduced with fast QPCR.
Figures

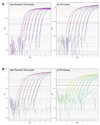
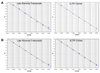
References
-
- Bushman F. Measuring covert HIV replication during HAART: the abundance of 2-LTR circles is not a reliable marker. Aids. 2003;17:749–750. - PubMed
-
- Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. - PubMed
-
- Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

