Mechanism-based inhibitors of MenE, an acyl-CoA synthetase involved in bacterial menaquinone biosynthesis
- PMID: 18762421
- PMCID: PMC2628629
- DOI: 10.1016/j.bmcl.2008.07.130
Mechanism-based inhibitors of MenE, an acyl-CoA synthetase involved in bacterial menaquinone biosynthesis
Abstract
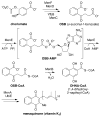
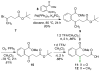
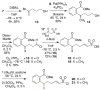
Menaquinone (vitamin K(2)) is an essential component of the electron transfer chain in many pathogens, including Mycobacterium tuberculosis and Staphylococcus aureus, and menaquinone biosynthesis is a potential target for antibiotic drug discovery. We report herein a series of mechanism-based inhibitors of MenE, an acyl-CoA synthetase that catalyzes adenylation and thioesterification of o-succinylbenzoic acid (OSB) during menaquinone biosynthesis. The most potent compound inhibits MenE with an IC(50) value of 5.7microM.
Figures
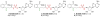

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

