ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65
- PMID: 18762583
- PMCID: PMC2528584
- DOI: 10.1083/jcb.200805045
ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65
Abstract
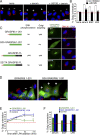
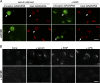
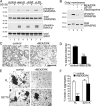
Directed cell migration requires the orientation of the Golgi and centrosome toward the leading edge. We show that stimulation of interphase cells with the mitogens epidermal growth factor or lysophosphatidic acid activates the extracellular signal-regulated kinase (ERK), which phosphorylates the Golgi structural protein GRASP65 at serine 277. Expression of a GRASP65 Ser277 to alanine mutant or a GRASP65 1-201 truncation mutant, neither of which can be phosphorylated by ERK, prevents Golgi orientation to the leading edge in a wound assay. We show that phosphorylation of GRASP65 with recombinant ERK leads to the loss of GRASP65 oligomerization and causes Golgi cisternal unstacking. Furthermore, preventing Golgi polarization by expressing mutated GRASP65 inhibits centrosome orientation, which is rescued upon disassembly of the Golgi structure by brefeldin A. We conclude that Golgi remodeling, mediated by phosphorylation of GRASP65 by ERK, is critical for the establishment of cell polarity in migrating cells.
Figures


References
-
- Barr, F.A., M. Puype, J. Vandekerckhove, and G. Warren. 1997. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 91:253–262. - PubMed
-
- Bartz, R., and J. Seemann. 2008. Mitotic regulation of SREBP and ATF6 by separation of the Golgi and ER. Cell Cycle. 7:2100–2105. - PubMed
-
- Cau, J., and A. Hall. 2005. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 118:2579–2587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

