The continuing saga of the marine polyether biotoxins
- PMID: 18763702
- PMCID: PMC2730226
- DOI: 10.1002/anie.200801696
The continuing saga of the marine polyether biotoxins
Abstract
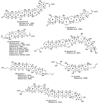
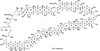
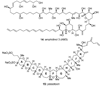
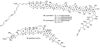
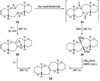
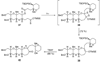
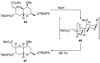
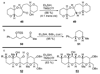
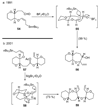
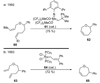
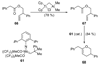
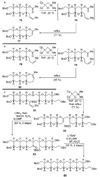
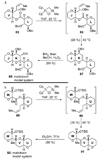
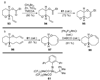
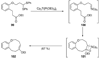
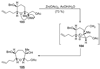
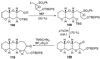
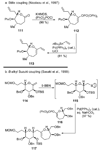
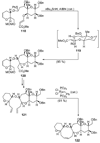
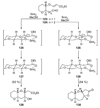
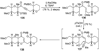
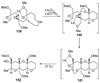
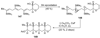
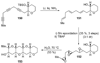
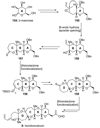
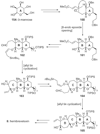
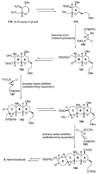
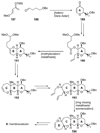
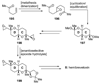
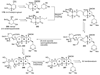
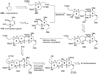
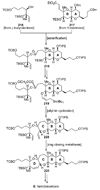
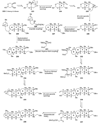
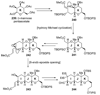
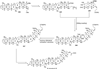
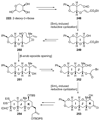
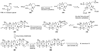
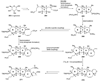
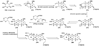
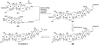
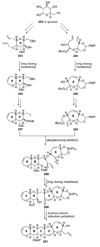
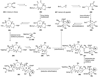
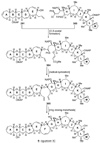
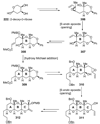
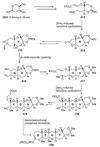
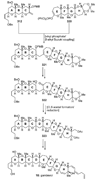
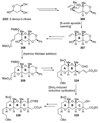
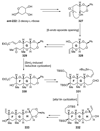
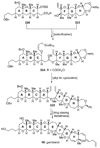
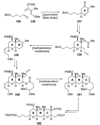
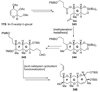
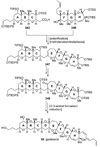
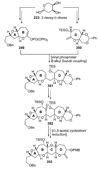
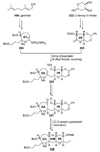
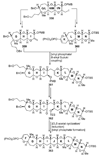
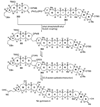
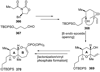
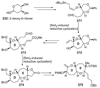
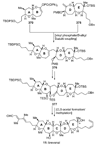
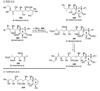
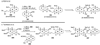
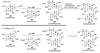
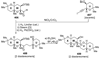
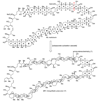
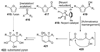
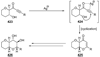
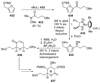
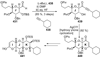
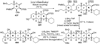
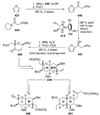
The unprecedented structure of the marine natural product brevetoxin B was elucidated by the research group of Nakanishi and Clardy in 1981. The ladderlike molecular architecture of this fused polyether molecule, its potent toxicity, and fascinating voltage-sensitive sodium channel based mechanism of action immediately captured the imagination of synthetic chemists. Synthetic endeavors resulted in numerous new methods and strategies for the construction of cyclic ethers, and culminated in several impressive total syntheses of this molecule and some of its equally challenging siblings. Of the marine polyethers, maitotoxin is not only the most complex and most toxic of the class, but is also the largest nonpolymeric natural product known to date. This Review begins with a brief history of the isolation of these biotoxins and highlights their biological properties and mechanism of action. Chemical syntheses are then described, with particular emphasis on new methods developed and applied to the total syntheses. The Review ends with a discussion of the, as yet unfinished, story of maitotoxin, and projects into the future of this area of research.
Figures








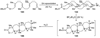



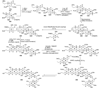
References
-
- Yasumoto T, Murata M. Chem. Rev. 1993;93:1897.
- Botana LM, editor. Phycotoxins: Chemistry and Biochemistry. Ames: Blackwell Publishing; 2007. p. 345.
-
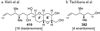
- Lin Y-Y, Risk M, Ray SM, Van Engen D, Clardy J, Golik J, James JC, Nakanishi K. J. Am. Chem. Soc. 1981;103:6773.
-
- Murata M, Legrand AM, Ishibashi Y, Fukui M, Yasumoto T. J. Am. Chem. Soc. 1990;112:4380.
-
- Yasumoto T. Chem. Rec. 2001;1:228. - PubMed
-
- Anderson DM. Sci. Am. 1994;8:62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

