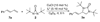Cu(I)-catalyzed asymmetric diamination of conjugated dienes
- PMID: 18763785
- PMCID: PMC2659555
- DOI: 10.1021/ol801605w
Cu(I)-catalyzed asymmetric diamination of conjugated dienes
Abstract
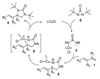
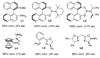
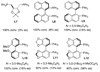
A Cu(I)-catalyzed asymmetric diamination for a variety of conjugated dienes and a triene with encouraging ee's has been effectively achieved using (R)-DTBM-SEGPHOS as a chiral ligand and di- tert-butyldiaziridinone as the nitrogen source.
Figures
References
-
-
For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580. Mortensen MS, O’Doherty GA. Chemtracts: Organic Chemistry. 2005;18:555. Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug. Des. 2006;67:101.
-
-
-
For examples of metal-mediated diaminations, see: Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979;962:962. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420. Muñiz K. Eur. J. Org. Chem. 2004:2243. Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163. Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.
-
-
-
For recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035.
-
-
-
For Rh(II)-, Fe(III)-catalyzed diamination with TsNCl2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem., Int. Ed. 2001;40:4277. Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777.
-
-
-
For a recent Pd(II)-catalyzed intermolecular diamination of conjugated dienes, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources