Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death
- PMID: 18764739
- PMCID: PMC2933567
- DOI: 10.1089/ars.2008.2263
Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death
Abstract
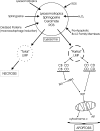
Lysosomes critically regulate the pH-dependent catabolism of extracellular and intracellular macromolecules delivered from the endocytic/heterophagy and autophagy pathways, respectively. The importance of lysosomes to cell survival is underscored not only by their unique ability effectively to degrade metalloproteins and oxidatively damaged macromolecules, but also by the distinct potential for induction of both caspase-dependent and -independent cell death with a compromise in the integrity of lysosome function. Oxidative stress and free radical damage play a principal role in cell death induced by lysosome dysfunction and may be linked to several upstream and downstream stimuli, including alterations in the autophagy degradation pathway, inhibition of lysosome enzyme function, and lysosome membrane damage. Neurons are sensitive to lysosome dysfunction, and the contribution of oxidative stress and free radical damage to lysosome dysfunction may contribute to the etiology of neurodegenerative disease. This review provides a broad overview of lysosome function and explores the contribution of oxidative stress and autophagy to lysosome dysfunction-induced neuron death. Putative signaling pathways that either induce lysosome dysfunction or result from lysosome dysfunction or both, and the role of oxidative stress, free radical damage, and lysosome dysfunction in pediatric lysosomal storage disorders (neuronal ceroid lipofuscinoses or NCL/Batten disease) and in Alzheimer's disease are emphasized.
Figures


References
-
- Adam-Vizi V. Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. - PubMed
-
- Adamec E. Mohan PS. Cataldo AM. Vonsattel JP. Nixon RA. Up-regulation of the lysosomal system in experimental models of neuronal injury: implications for Alzheimer's disease. Neuroscience. 2000;100:663–675. - PubMed
-
- Amano T. Nakanishi H. Kondo T. Tanaka T. Oka M. Yamamoto K. Age-related changes in cellular localization and enzymatic activities of cathepsins B, L and D in the rat trigeminal ganglion neuron. Mech Ageing Dev. 1995;83:133–141. - PubMed
-
- Ambroso JL. Harris C. Chloroquine accumulation and alterations of proteolysis and pinocytosis in the rat conceptus in vitro. Biochem Pharmacol. 1994;47:679–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

