Inteferons pen the JAK-STAT pathway
- PMID: 18765289
- PMCID: PMC2741134
- DOI: 10.1016/j.semcdb.2008.08.010
Inteferons pen the JAK-STAT pathway
Abstract
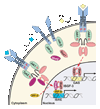
Characterization of how interferons (IFNs) mediate their biological response led to identification of the JAK-STAT signaling cascade, where JAKs are receptor-associated kinases and STATs the transcription factors they activate. Today, 4 JAKs and 7 STATs are known to transduce pivotal signals for the over 50 members of the four-helix bundle family of cytokines. This review will provide an overview and historical perspective of the JAK-STAT paradigm.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

