Vessel-specific Toll-like receptor profiles in human medium and large arteries
- PMID: 18765390
- PMCID: PMC2748975
- DOI: 10.1161/CIRCULATIONAHA.108.789172
Vessel-specific Toll-like receptor profiles in human medium and large arteries
Abstract
Background: Inflammatory vasculopathies, ranging from the vasculitides (Takayasu arteritis, giant cell arteritis, and polyarteritis nodosa) to atherosclerosis, display remarkable target tissue tropisms for selected vascular beds. Molecular mechanisms directing wall inflammation to restricted anatomic sites within the vascular tree are not understood. We have examined the ability of 6 different human macrovessels (aorta and subclavian, carotid, mesenteric, iliac, and temporal arteries) to initiate innate and adaptive immune responses by comparing pathogen-sensing and T-cell-stimulatory capacities.
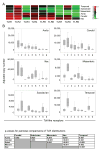
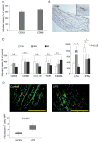
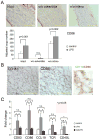
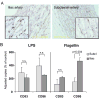
Methods and results: Gene expression analysis for pathogen-sensing Toll-like receptors (TLRs) 1 to 9 showed vessel-specific profiles, with TLR2 and TLR4 ubiquitously present, TLR7 and TLR9 infrequent, and TLR1, TLR3, TLR5, TLR6, and TLR8 expressed in selective patterns. Experiments with vessel walls stripped of the intimal or adventitial layer identified dendritic cells at the media-adventitia junction as the dominant pathogen sensors. In human artery-severe combined immunodeficiency (SCID) mouse chimeras, adoptively transferred human T cells initiated vessel wall inflammation if wall-embedded dendritic cells were conditioned with TLR ligands. Wall-infiltrating T cells displayed vessel-specific activation profiles with differential production of CD40L, lymphotoxin-alpha, and interferon-gamma. Vascular bed-specific TLR fingerprints were functionally relevant, as exemplified by differential responsiveness of iliac and subclavian vessels to TLR5 but not TLR4 ligands.
Conclusions: Populated by indigenous dendritic cells, medium and large human arteries have immune-sensing and T-cell-stimulatory functions. Each vessel in the macrovascular tree exhibits a distinct TLR profile and supports selective T-cell responses, imposing vessel-specific risk for inflammatory vasculopathies.
Figures


References
-
- Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20:409–421. - PubMed
-
- Zeek PM. Periarteritis nodosa; a critical review. Am J Clin Pathol. 1952;22:777–790. - PubMed
-
- Morgan MD, Savage CO. Vasculitis in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2005;19:215–233. - PubMed
-
- Burke AP, Tavora F, Narula N, Tomaszewski JE, Virmani R. Aortitis and ascending aortic aneurysm: description of 52 cases and proposal of a histologic classification. Hum Pathol. 2008;39:514–526. - PubMed
-
- Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

