Effect of illicit direct to consumer advertising on use of etanercept, mometasone, and tegaserod in Canada: controlled longitudinal study
- PMID: 18765444
- PMCID: PMC2528895
- DOI: 10.1136/bmj.a1055
Effect of illicit direct to consumer advertising on use of etanercept, mometasone, and tegaserod in Canada: controlled longitudinal study
Abstract
Objective: To assess the impact of direct to consumer advertising of prescription drugs in the United States on Canadian prescribing rates for three heavily marketed drugs-etanercept, mometasone, and tegaserod.
Design: Controlled quasi-experimental study using interrupted time series analysis.
Population: Representative sample of 2700 Canadian pharmacies and prescription data from 50 US Medicaid programmes.
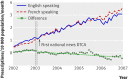
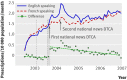
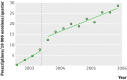
Main outcome measures: Differences in number of filled prescriptions per 10,000 population per month between English speaking and French speaking (control) Canadian provinces before and after the start of direct to consumer advertising in the United States.
Results: Spending on direct to consumer advertising for study drugs ranged from $194m to $314m ( pound104m- pound169m; euro131m-euro212m) over the study period. Prescription rates for etanercept and mometasone did not increase in English speaking provinces relative to French speaking controls after the start of direct to consumer advertising. In contrast, tegaserod prescriptions increased 42% (0.56 prescriptions/10,000 residents, 95% confidence interval 0.37 to 0.76) in English speaking provinces immediately after the start of US direct to consumer advertising. Uncontrolled analysis of US Medicaid data showed a larger 56% increase in tegaserod prescriptions. However, this increase did not persist over time in either country, despite continued advertising.
Conclusions: Exposure to US direct to consumer advertising transiently influenced both Canadian and US prescribing rates for tegaserod, a drug later withdrawn owing to safety concerns. The impact of direct to consumer advertising on drug use seems to be highly variable and probably depends on the characteristics of the advertised drug, the level of exposure to direct to consumer advertising, and the cultural context.
Conflict of interest statement
Competing interests: None declared.
Figures

Comment in
-
Direct to consumer advertising of prescription drugs.BMJ. 2008 Sep 2;337:a985. doi: 10.1136/bmj.a985. BMJ. 2008. PMID: 18765449 No abstract available.
References
-
- Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med 2007;357:673-81. - PubMed
-
- Holmer AF. Direct-to-consumer prescription drug advertising builds bridges between patients and physicians. JAMA 1999;281:380-2. - PubMed
-
- Hollon MF. Direct-to-consumer advertising: a haphazard approach to health promotion. JAMA 2005;293:2030-33. - PubMed
-
- Cassels A. Canada may be forced to allow direct to consumer advertising. BMJ 2006, 10.1136/bmj.332.7556.1469-a - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
