Mechanism for improved insulin sensitivity after gastric bypass surgery
- PMID: 18765510
- PMCID: PMC2729236
- DOI: 10.1210/jc.2008-1030
Mechanism for improved insulin sensitivity after gastric bypass surgery
Abstract
Context: Surgical treatments of obesity have been shown to induce rapid and prolonged improvements in insulin sensitivity.
Objective: The aim of the study was to investigate the effects of gastric bypass surgery and the mechanisms that explain the improvement in insulin sensitivity.
Design: We performed a cross-sectional, nonrandomized, controlled study.
Setting: This study was conducted jointly between the Departments of Exercise Science and Physiology at East Carolina University in Greenville, North Carolina.
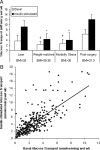
Subjects: Subjects were recruited into four groups: 1) lean [body mass index (BMI) < 25 kg/m(2); n = 93]; 2) weight-matched (BMI = 25 to 35 kg/m(2); n = 310); 3) morbidly obese (BMI > 35 kg/m(2); n = 43); and 4) postsurgery patients (BMI approximately 30 kg/m(2); n = 40). Postsurgery patients were weight stable 1 yr after surgery.
Main outcome measures: Whole-body insulin sensitivity, muscle glucose transport, and muscle insulin signaling were assessed.
Results: Postsurgery subjects had insulin sensitivity index values that were similar to the lean and higher than morbidly obese and weight-matched control subjects. Glucose transport was higher in the postsurgery vs. morbidly obese and weight-matched groups. IRS1-pSer(312) in the postsurgery group was lower than morbidly obese and weight-matched groups. Inhibitor kappaBalpha was higher in the postsurgery vs. the morbidly obese and weight-matched controls, indicating reduced inhibitor of kappaB kinase beta activity.
Conclusions: Insulin sensitivity and glucose transport are greater in the postsurgery patients than predicted from the weight-matched group, suggesting that improved insulin sensitivity after bypass is due to something other than, or in addition to, weight loss. Improved insulin sensitivity is related to reduced inhibitor of kappaB kinase beta activity and enhanced insulin signaling in muscle.
Figures



References
-
- Pories WJ 1992 Why does the gastric bypass control type 2 diabetes mellitus? Obes Surg 2:303–313 - PubMed
-
- Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M 2008 Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323 - PubMed
-
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K 2004 Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

