Clathrin-independent endocytosis of ErbB2 in geldanamycin-treated human breast cancer cells
- PMID: 18765569
- PMCID: PMC2707784
- DOI: 10.1242/jcs.020404
Clathrin-independent endocytosis of ErbB2 in geldanamycin-treated human breast cancer cells
Abstract
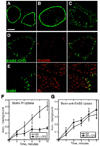
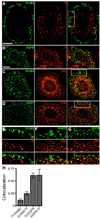
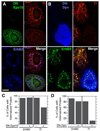
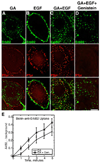
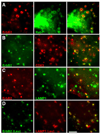
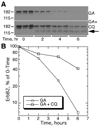
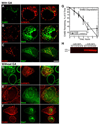
The epidermal growth factor (EGF)-receptor family member ErbB2 is commonly overexpressed in human breast cancer cells and correlates with poor prognosis. Geldanamycin (GA) induces the ubiquitylation, intracellular accumulation and degradation of ErbB2. Whether GA stimulates ErbB2 internalization is controversial. We found that ErbB2 was internalized constitutively at a rate that was not affected by GA in SK-BR-3 breast cancer cells. Instead, GA treatment altered endosomal sorting, causing the transport of ErbB2 to lysosomes for degradation. In contrast to earlier work, we found that ErbB2 internalization occurred by a clathrin- and tyrosine-kinase-independent pathway that was not caveolar, because SK-BR-3 cells lack caveolae. Similar to cargo of the glycosylphosphatidylinositol (GPI)-anchored protein-enriched early endosomal compartment (GEEC) pathway, internalized ErbB2 colocalized with cholera toxin B subunit, GPI-anchored proteins and fluid, and was often seen in short tubules or large vesicles. However, in contrast to the GEEC pathway in other cells, internalization of ErbB2 and fluid in SK-BR-3 cells did not require Rho-family GTPase activity. Accumulation of ErbB2 in vesicles containing constitutively active Arf6-Q67L occurred only without GA treatment; Arf6-Q67L did not slow transport to lysosomes in GA-treated cells. Further characterization of this novel clathrin-, caveolae- and Rho-family-independent endocytic pathway might reveal new strategies for the downregulation of ErbB2 in breast cancer.
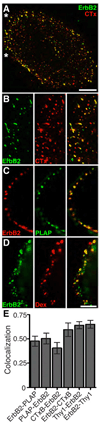
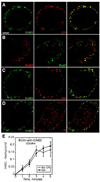
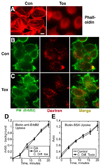
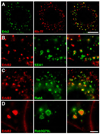
Figures
References
-
- Arreaza G, Melkonian KA, LaFevre-Bernt M, Brown DA. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src-family protein tyrosine kinase p62yes. J. Biol. Chem. 1994;269:19123–19127. - PubMed
-
- Baulida J, Kraus MH, Alimandi M, DiFiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 1996;271:5251–5257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

