Anaplasma phagocytophilum increases cathepsin L activity, thereby globally influencing neutrophil function
- PMID: 18765732
- PMCID: PMC2573316
- DOI: 10.1128/IAI.00851-08
Anaplasma phagocytophilum increases cathepsin L activity, thereby globally influencing neutrophil function
Abstract
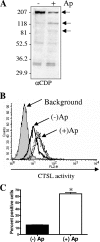
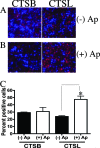
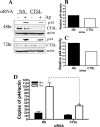
Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, is an unusual obligate intracellular pathogen that persists in neutrophils. A. phagocytophilum increases the binding of a repressor, CCAAT displacement protein (CDP), to the gp91(phox) promoter, thereby diminishing the host oxidative burst. We now show that A. phagocytophilum infection also enhances the binding of CDP to the promoters of human neutrophil peptide 1 and C/EBPepsilon--molecules important for neutrophil defense and maturation--suggesting that this is a general strategy used by this pathogen to alter polymorphonuclear leukocyte function. To explore the mechanism by which A. phagocytophilum increases CDP activity, we assessed the effects of this microbe on cathepsin L, a protease that cleaves CDP into a form with increased DNA binding ability. A. phagocytophilum infection resulted in elevated cathepsin L activity and the proteolysis of CDP. Blocking the action of cathepsin L with a chemical inhibitor or small interfering RNA targeting of this molecule caused a marked reduction in the degree of A. phagocytophilum infection. These data demonstrate that increasing cathepsin L activity is a strategy used by A. phagocytophilum to alter CDP activity and thereby globally influence neutrophil function. As therapeutic options for A. phagocytophilum and related organisms are limited, these results also identify a cellular pathway that may be targeted for the treatment of A. phagocytophilum infection.
Figures


Similar articles
-
Anaplasma phagocytophilum modulates gp91phox gene expression through altered interferon regulatory factor 1 and PU.1 levels and binding of CCAAT displacement protein.Infect Immun. 2005 Jan;73(1):208-18. doi: 10.1128/IAI.73.1.208-218.2005. Infect Immun. 2005. PMID: 15618156 Free PMC article.
-
Binding of Host Cell Surface Protein Disulfide Isomerase by Anaplasma phagocytophilum Asp14 Enables Pathogen Infection.mBio. 2020 Jan 28;11(1):e03141-19. doi: 10.1128/mBio.03141-19. mBio. 2020. PMID: 31992623 Free PMC article.
-
Epigenetic silencing of host cell defense genes enhances intracellular survival of the rickettsial pathogen Anaplasma phagocytophilum.PLoS Pathog. 2009 Jun;5(6):e1000488. doi: 10.1371/journal.ppat.1000488. Epub 2009 Jun 19. PLoS Pathog. 2009. PMID: 19543390 Free PMC article.
-
Invasion and survival strategies of Anaplasma phagocytophilum.Cell Microbiol. 2003 Nov;5(11):743-54. doi: 10.1046/j.1462-5822.2003.00323.x. Cell Microbiol. 2003. PMID: 14531890 Review.
-
Human granulocytic anaplasmosis and Anaplasma phagocytophilum.Emerg Infect Dis. 2005 Dec;11(12):1828-34. doi: 10.3201/eid1112.050898. Emerg Infect Dis. 2005. PMID: 16485466 Free PMC article. Review.
Cited by
-
Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum.Clin Microbiol Rev. 2011 Jul;24(3):469-89. doi: 10.1128/CMR.00064-10. Clin Microbiol Rev. 2011. PMID: 21734244 Free PMC article. Review.
-
Establishment of Host-Algal Endosymbioses: Genetic Response to Symbiont Versus Prey in a Sponge Host.Genome Biol Evol. 2021 Nov 5;13(11):evab252. doi: 10.1093/gbe/evab252. Genome Biol Evol. 2021. PMID: 34791195 Free PMC article.
-
The role of cysteine proteinases and their inhibitors in the host-pathogen cross talk.Curr Protein Pept Sci. 2012 Dec;13(8):767-75. doi: 10.2174/138920312804871102. Curr Protein Pept Sci. 2012. PMID: 23305363 Free PMC article. Review.
-
Colonization state influences the hemocyte proteome in a beneficial squid-Vibrio symbiosis.Mol Cell Proteomics. 2014 Oct;13(10):2673-86. doi: 10.1074/mcp.M113.037259. Epub 2014 Jul 18. Mol Cell Proteomics. 2014. PMID: 25038065 Free PMC article.
-
The Role of Hemocytes in the Hawaiian Bobtail Squid, Euprymna scolopes: A Model Organism for Studying Beneficial Host-Microbe Interactions.Front Microbiol. 2017 Jan 6;7:2013. doi: 10.3389/fmicb.2016.02013. eCollection 2016. Front Microbiol. 2017. PMID: 28111565 Free PMC article. Review.
References
-
- Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 1643946-3949. - PubMed
-
- Carlyon, J. A., and E. Fikrig. 2006. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr. Opin. Hematol. 1328-33. - PubMed
-
- Catt, D., W. Luo, and D. G. Skalnik. 1999. DNA-binding properties of CCAAT displacement protein cut repeats. Cell. Mol. Biol. (Noisy-le-Grand) 451149-1160. - PubMed
-
- Coqueret, O., G. Berube, and A. Nepveu. 1996. DNA binding by cut homeodomain proteins is down-modulated by protein kinase C. J. Biol. Chem. 27124862-24868. - PubMed
-
- Coqueret, O., N. Martin, G. Berube, M. Rabbat, D. W. Litchfield, and A. Nepveu. 1998. DNA binding by cut homeodomain proteins is down-modulated by casein kinase II. J. Biol. Chem. 2732561-2566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

