Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice
- PMID: 18765787
- PMCID: PMC2749675
- DOI: 10.1101/gad.1686208
Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice
Abstract
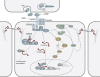
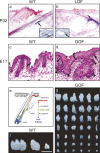
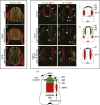
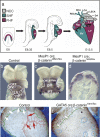
Wnt signaling is one of a handful of powerful signaling pathways that play crucial roles in the animal life by controlling the genetic programs of embryonic development and adult homeostasis. When disrupted, these signaling pathways cause developmental defects, or diseases, among them cancer. The gateway of the canonical Wnt pathway, which contains >100 genes, is an essential molecule called beta-catenin (Armadillo in Drosophila). Conditional loss- and gain-of-function mutations of beta-catenin in mice provided powerful tools for the functional analysis of canonical Wnt signaling in many tissues and organs. Such studies revealed roles of Wnt signaling that were previously not accessible to genetic analysis due to the early embryonic lethality of conventional beta-catenin knockout mice, as well as the redundancy of Wnt ligands, receptors, and transcription factors. Analysis of conditional beta-catenin loss- and gain-of-function mutant mice demonstrated that canonical Wnt signals control progenitor cell expansion and lineage decisions both in the early embryo and in many organs. Canonical Wnt signaling also plays important roles in the maintenance of various embryonic or adult stem cells, and as recent findings demonstrated, in cancer stem cell types. This has opened new opportunities to model numerous human diseases, which have been associated with deregulated Wnt signaling. Our review summarizes what has been learned from genetic studies of the Wnt pathway by the analysis of conditional beta-catenin loss- and gain-of-function mice.
Figures




References
-
- Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. Assembly of the cadherin–catenin complex in vitro with recombinant proteins. J. Cell Sci. 1994;107:3655–3663. - PubMed
-
- Abu-Elmagd M., Garcia-Morales C., Wheeler G.N. Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev. Biol. 2006;298:285–298. - PubMed
-
- Ahn K., Mishina Y., Hanks M.C., Behringer R.R., Crenshaw E.B. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development. 2001;128:4449–4461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
